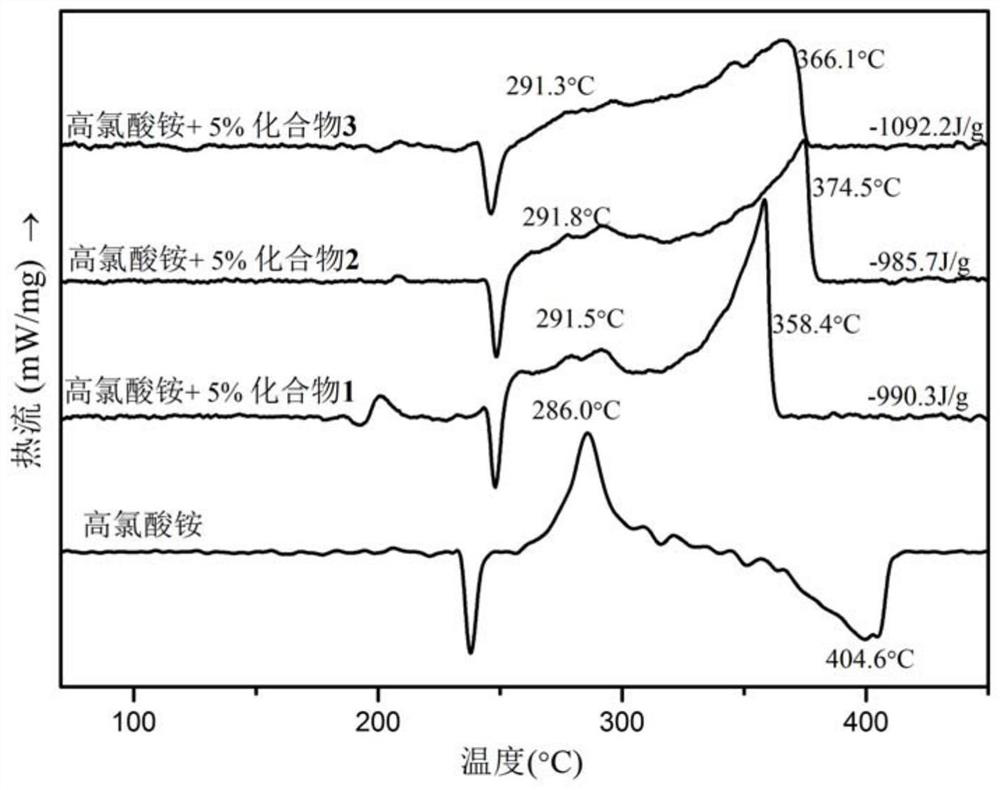
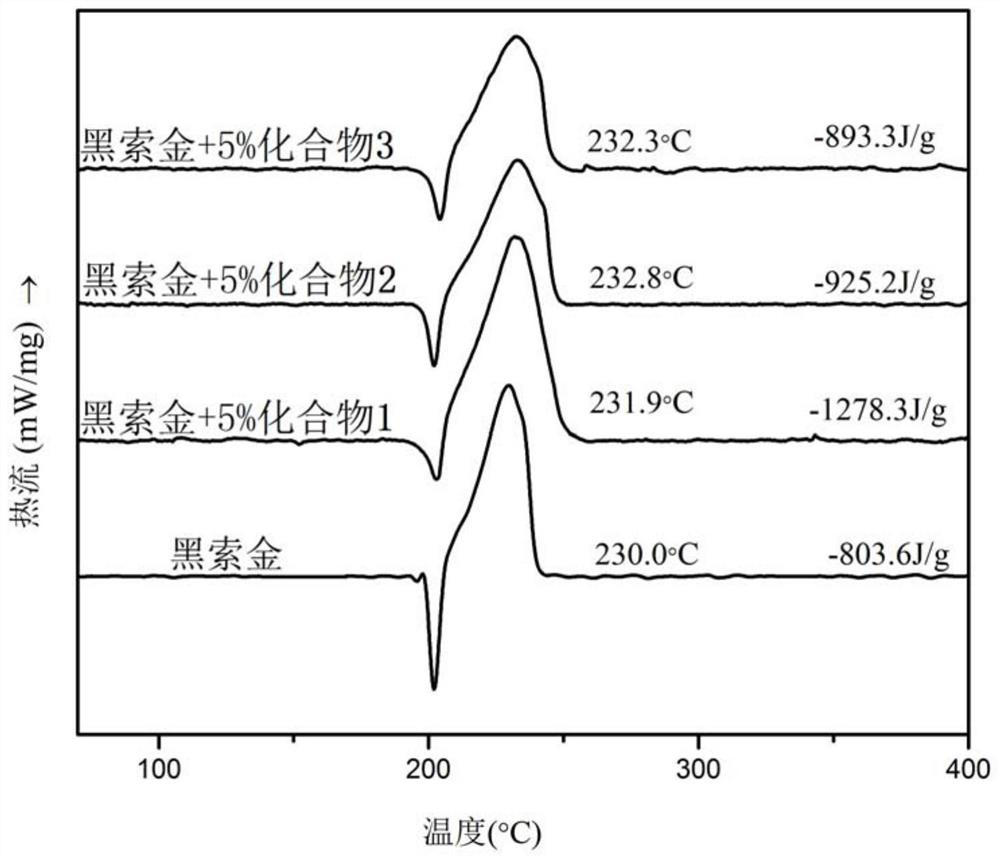
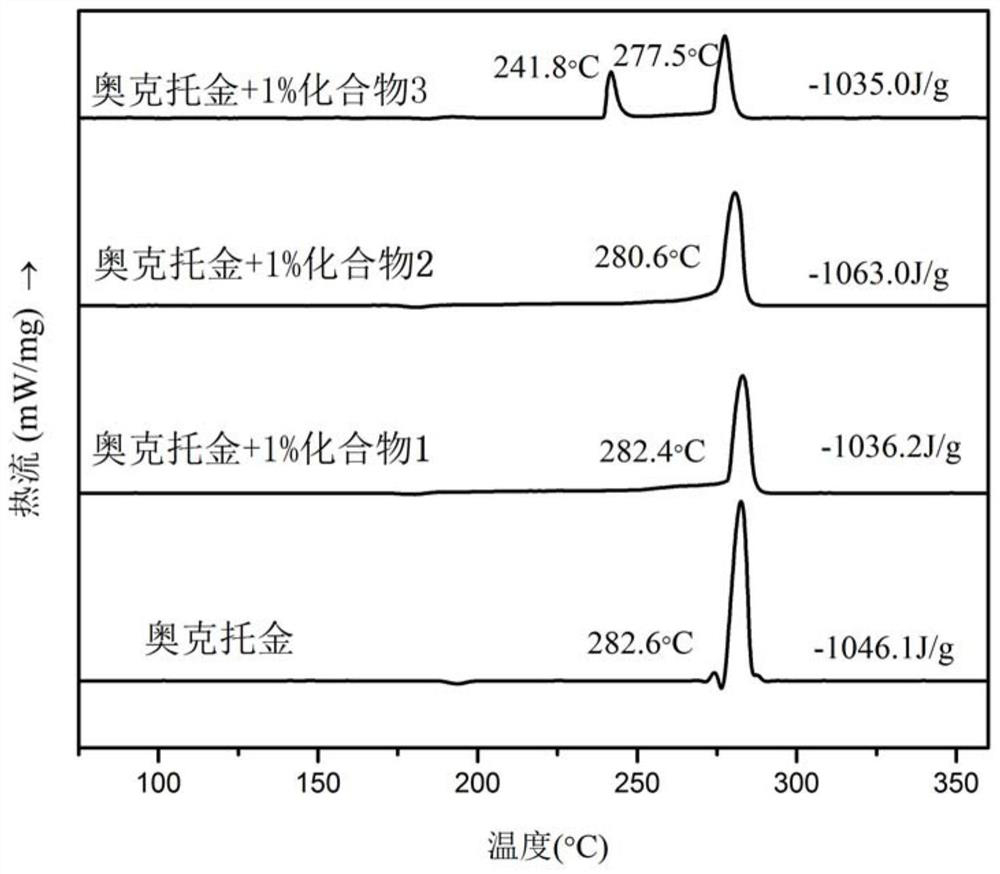
Ferrocene picrate ionic compound and preparation method thereof
A technology of ionic compounds and picrates, applied in chemical instruments and methods, metallocenes, organic chemistry, etc., can solve the problems of easy volatility, easy migration, energy-free, etc., to change the speed of chemical reactions and overcome the cumbersome synthesis process , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 50mL round bottom flask, add 0.93g (5.0mmol) ferrocene, 0.405g (15.0mmol) aluminum powder, 2g (15.0mmol) anhydrous aluminum trichloride, 5mL chlorobenzene, stir evenly with a magnetic force, and heat the oil bath to 130°C, reflux for 4 hours, transfer to an ice-water bath to fully cool, and in the state of continuous stirring, slowly inject 2mL of methanol into the flask in batches through the condenser, and then pour 20mL of ice water into the flask, and wait until the reaction solution is no longer exothermic And after fully cooling, filter under reduced pressure to remove aluminum powder, carry out liquid separation to filtrate, separate aqueous phase extract 3 times with cyclohexane (each 10mL), obtain [cyclopentadiene-iron-chlorobenzene] tetrachloro Aluminum salt, the reaction equation is as follows:
[0022]
[0023] After dissolving 1.255g (5.0mmol) sodium picrate in 2.5mL of distilled water, add into the extracted water phase in the dark, at this time, a...
Embodiment 2
[0027] Add 0.461g (1.0mmol) [cyclopentadiene-iron-chlorobenzene] picrate, 0.287g (1.25mmol) picric acid, 0.382g (2.77mmol) potassium carbonate, 5mLN,N- Dimethylformamide was stirred at room temperature in the dark for 2 hours, and then 5mL of ice water was added to the reaction flask. At this time, a large amount of dark yellow solids could be seen to precipitate, filtered under reduced pressure, and the filter cake was rinsed with absolute ethanol. Dried in a vacuum oven at 40°C, the yellow flocculent solid product [cyclopentadiene-iron-2,4,6-trinitrodiphenyl ether] picrate was obtained, with a yield of 82.4%, and the reaction equation was as follows :
[0028]
[0029] The above-mentioned [cyclopentadiene-iron-2,4,6-trinitrodiphenyl ether] picrate has very little solubility in water and can be dissolved in acetonitrile, acetone, and DMSO, and its structural characterization data is: 1 H NMR (400MHz, CDCl 3 -d 6 )δ: 8.59(s,4H), 6.83(s,2H), 6.48(s,2H), 6.35(s,1H), 5.23(s...
Embodiment 3
[0031] Add 0.461g (1.0mmol) [cyclopentadiene-iron-chlorobenzene] picrate, 0.306g (1.25mmol) trinitroresorcinol, 0.382g (2.77mmol) potassium carbonate to a 10mL brown reaction bottle , 5mL N,N-dimethylformamide, after stirring for 2 hours in the dark at room temperature, a large amount of dark yellow solids can be seen to precipitate, then add 5mL ice water to the reaction bottle, let stand and filter under reduced pressure, filter cake with a small amount of After rinsing with absolute ethanol, dry it in a vacuum oven at 40°C to obtain a yellow solid product [cyclopentadiene-iron-2,4,6-trinitro-3-hydroxydiphenyl ether] picrate, Its productive rate is 62.6%, and reaction equation is as follows:
[0032]
[0033] The above-mentioned [cyclopentadiene-iron-2,4,6-trinitro-3-hydroxydiphenyl ether] picrate has very little solubility in water, and can be dissolved in ethanol, acetonitrile, acetone, DMSO, and its structural characterization The data is: 1 H NMR (400MHz, CDCl 3 -d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com