Method for preparing florfenicol-chitosan/long-chain carboxylic acid nano-micelle freeze-dried powder
A long-chain carboxylic acid and nano-micelle technology, which is applied in freeze-drying transportation, powder transportation, pharmaceutical formulations, etc., can solve the problems of low bioavailability of common preparations, high cost of use, wide application, and difficulty in forming complexes. Achieve huge social and economic benefits, wide applicability, good research and development and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
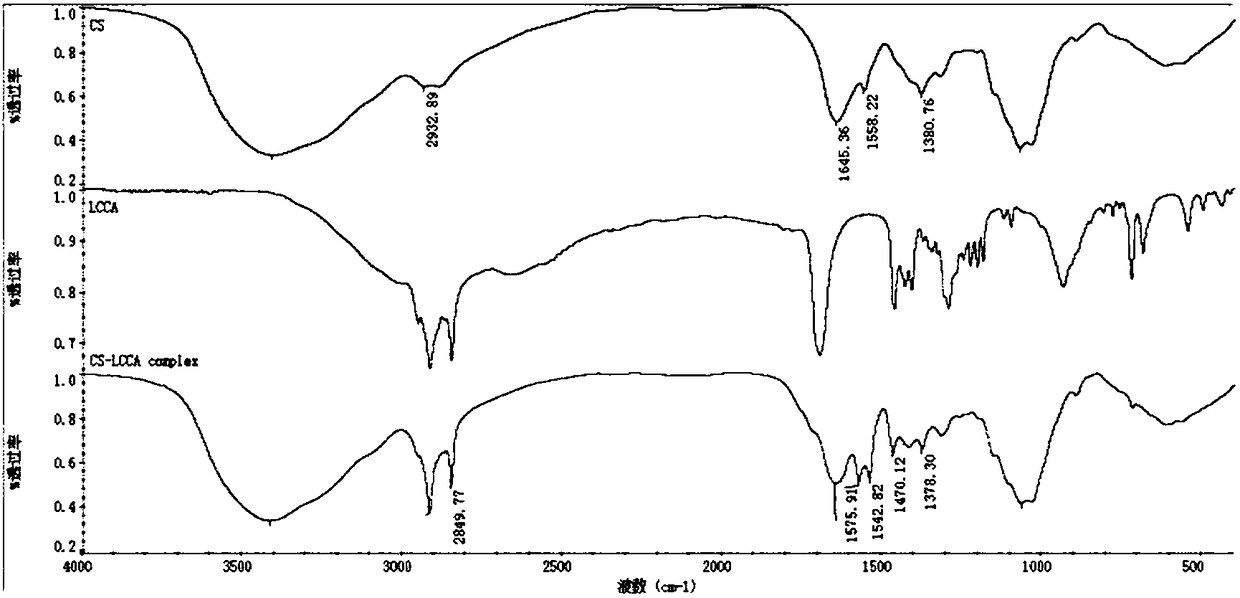
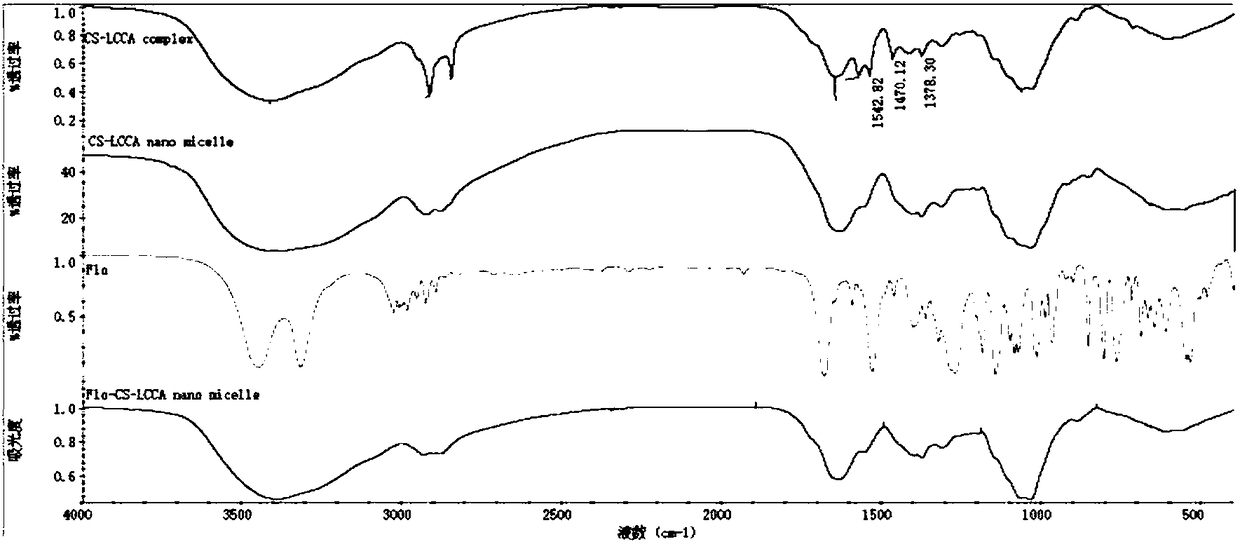
[0038]First, polyvinyl alcohol-grafted aminopolysaccharide was added into deionized water to completely dissolve to obtain solution I, and then palmitic acid was added to ethanol to dissolve to obtain solution II, and solution I and solution II were mixed, and then the probe was ultrasonically treated and injected into the solution. Wherein add DCC, HOBt catalyst, DCC and HOBT are mixed according to mass ratio (1-3):(2-6), select DCC and HOBT to mix according to mass ratio 1:2 in this embodiment, add pH adjustment reagent to control system acidity and alkalinity pH 1. Control the ultrasonic output power to 10w, the ultrasonic time to 12s, the interval time to 4s, and the number of ultrasonics to 150 times; by controlling the reaction temperature at 5°C and the reaction time at 2h, the polyvinyl alcohol-grafted aminopolysaccharide-palmitic acid self-assembled nanomicelles and The catalyst mixture is separated, dialyzed, freeze-dried and washed to obtain polyvinyl alcohol-grafted...
Embodiment 2
[0040] First add amino polysaccharide to deionized water to completely dissolve to prepare solution I, then add conjugated linoleic acid to dissolve in low-concentration acetic acid to prepare solution II, mix solution I and solution II, then perform ultrasonic treatment on the probe and inject Wherein add EDC, NHS catalyst, EDC and NHS mix according to mass ratio (1-3):(2-6), choose EDC and NHS mix according to mass ratio 2:5 in this embodiment, add pH adjusting reagent control system pH 6. Control the ultrasonic output power to 100w, the ultrasonic time to 10s, the interval time to 10s, and the number of ultrasonics to 100 times; by controlling the reaction temperature to 35°C and the reaction time to 1.5h, amino polysaccharide-conjugated linoleic acid self-assembled nanomicelles and catalysts can be obtained The mixed solution was separated, dialyzed, freeze-dried and washed to obtain aminopolysaccharide-conjugated linoleic acid nanomicelles. Add it to low-concentration ace...
Embodiment 3
[0046] First add the amino polysaccharide quaternary ammonium salt into deionized water to completely dissolve to obtain solution I, then add cis-15-tetradecenoic acid to dissolve in isopropanol to obtain solution II, and mix solution I and solution II , then carry out probe sonication and add PyBop, DIEA catalyst wherein, PyBop and DIEA are mixed according to mass ratio (1-3):(2-6), select PyBop and DIEA to mix according to mass ratio 1:3 in this embodiment, Add a pH adjusting reagent to control the pH of the system to 12, control the ultrasonic output power to 50w, the ultrasonic time to 8s, the interval time to 10s, and the number of ultrasonics to 100 times; by controlling the reaction temperature to 50°C and the reaction time to 1.8h, the amino polysaccharide-cis-15- The mixed solution of tetracosenoic acid self-assembled nanometer micelles and catalyst is separated, dialyzed, freeze-dried and washed to obtain aminopolysaccharide-cis-15-tetracosenoic acid nanometer micelle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com