Method for preparing catalyst for preparing secondary amines by hydrogenation of nitrile compounds, catalyst product and application of catalyst product
A technology of catalysts and compounds, applied in the preparation of catalysts for the hydrogenation of nitrile compounds to secondary amines and its products and applications, can solve the problems of waste of raw materials, no obvious economic advantages, equipment corrosion, etc., and achieve process environmental protection, Excellent catalytic performance, less poisonous effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
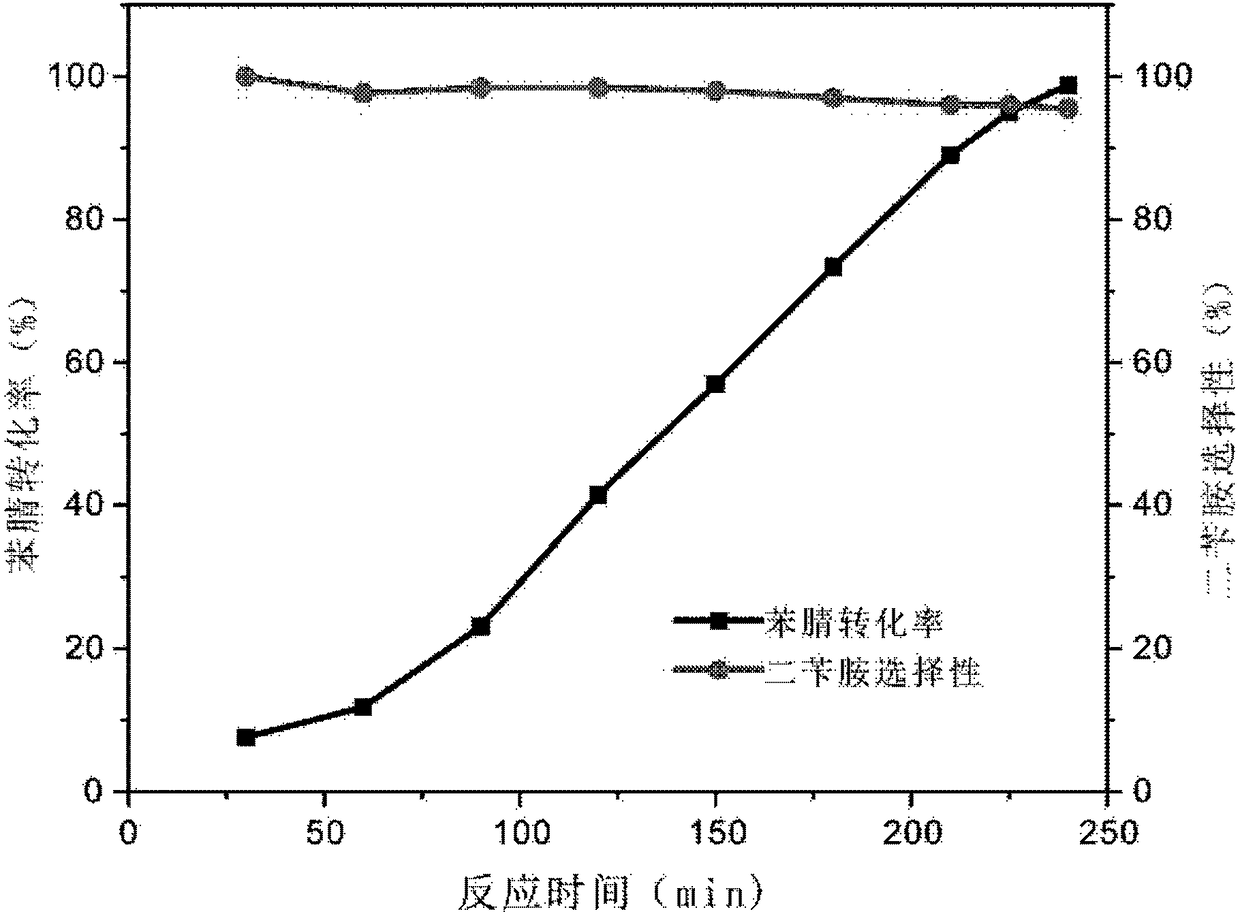
[0073] Example 1: Preparation of 3Ni-Pd / C catalyst and its activity test in benzonitrile hydrogenation reaction
[0074] Preparation of 3Ni-Pd / C catalyst:
[0075] First, it is synthesized by impregnation method, and Pd is loaded on the activated carbon carrier (Pd / C), and then the nickel oxide additive is deposited (total 3 deposition cycles) on the Pd / C sample by atomic layer deposition method, A 3Ni-Pd / C catalyst is obtained.
[0076] Specifically, add 150ml of deionized water to a 250ml beaker, add 10ml of pre-configured 0.02mol / L PdCl 2 Hydrochloric acid solution (wherein the concentration of hydrochloric acid is about 0.1mol / L, the purpose is to increase the PdCl 2 Solubility of solids) and 350 mg of sodium citrate dihydrate (as a stabilizer to help better control particle size). After uniform magnetic stirring, 400 mg of activated carbon carrier (Sigma Aldrich) was added, ultrasonically stirred for 20 min to disperse the activated carbon in water, and stirred at room...
Embodiment 2
[0083] Embodiment 2: Preparation of 3Ni-Pd / C catalyst and its activity test in nitriles hydrogenation reaction
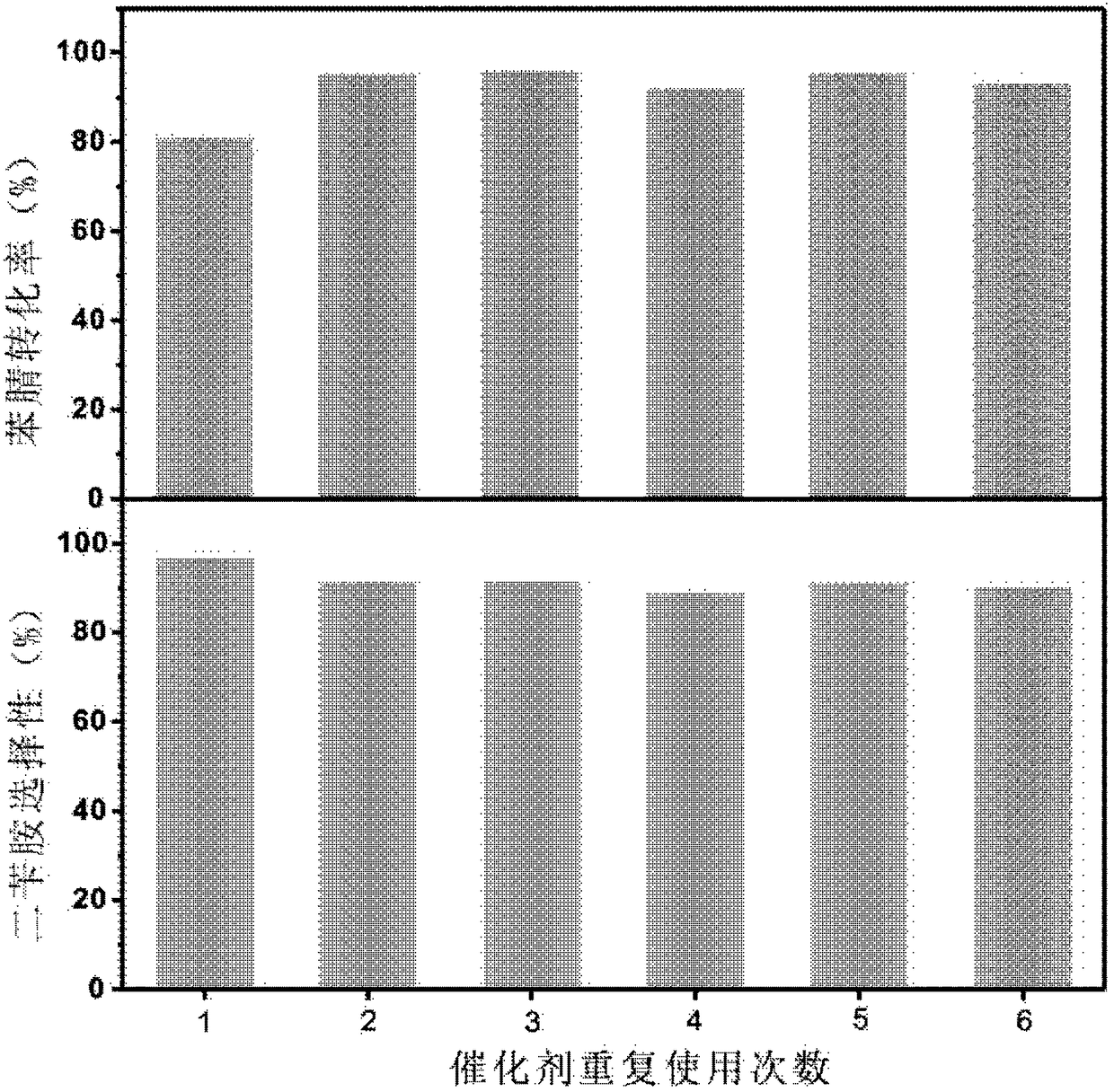
[0084] The 3Ni-Pd / C catalyst described in Example 1 was used to catalyze the hydrogenation of different nitrile reaction substrates to prepare the corresponding secondary amines. The reaction test procedure is similar to the activity test 1 part in the implementation example 1, but one or more conditions in the substrate type, temperature and substrate consumption have been changed, and the analysis results are shown in Table 2. Table 2 shows the reaction results of the 3Ni-Pd / C catalyst prepared according to Example 1 of the present invention in Example 2 to catalyze the hydrogenation of different nitriles to prepare corresponding secondary amines. As can be seen from this table 2, utilize the high-efficiency catalyst prepared by the present invention, for ten kinds of different nitriles (relate to aliphatic nitrile and aromatic nitrile), all can obtain correspondi...
Embodiment 3
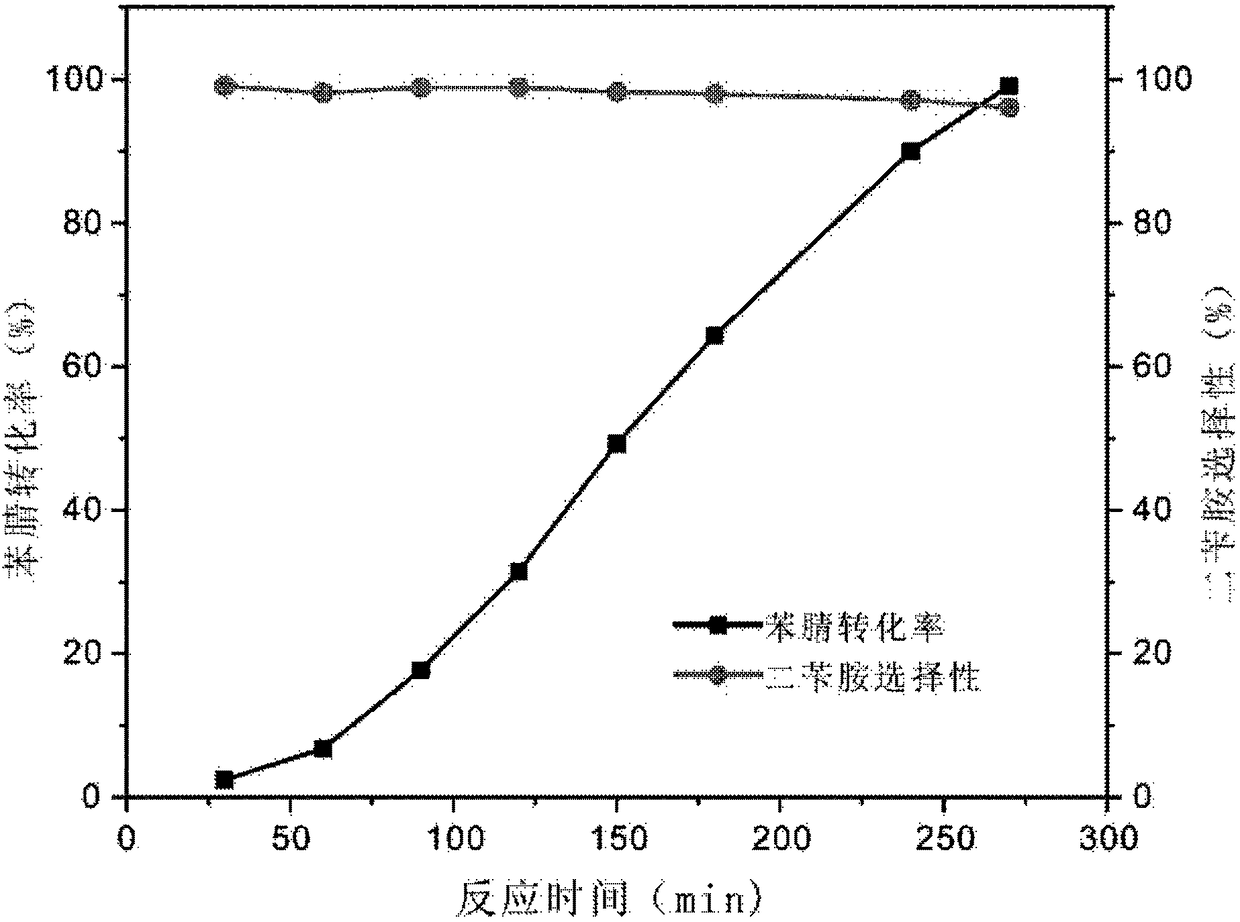
[0087] Example 3: Preparation of 5Ni-Pd / C catalyst and its activity test in benzonitrile hydrogenation reaction
[0088] Preparation of 5Ni-Pd / C catalyst: Firstly, it was synthesized by impregnation method, and Pd was loaded on the activated carbon support (Pd / C), which was the same as in Example 1. Then use the atomic layer deposition method to deposit the nickel oxide additive on the Pd / C sample. The deposition method is the same as that of Example 1, except that the deposition cycle is changed to 5 cycles to obtain a 5Ni-Pd / C catalyst. According to the elemental analysis test (ICP-AES), the mass content of Pd is 4.5 wt%, and the mass content of the nickel oxide additive is 2.5 wt%. According to the results of high-resolution electron microscopy (JEM-2100F), the particle size of Pd is 2-3nm.
[0089] Activity test for catalytic hydrogenation of benzonitrile: The 5Ni-Pd / C catalyst obtained above was pretreated before the reaction. Catalyst pretreatment: first in 10% O 2 / H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com