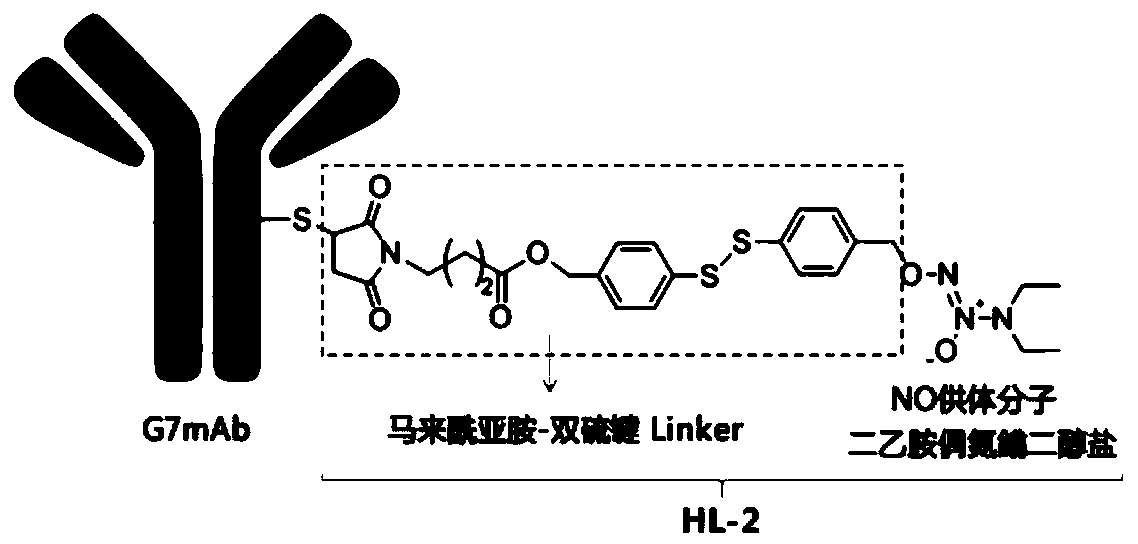
A conjugate of monoclonal antibody targeting CD24 and diethylaminoazonium dialkoxide and its application
A technology of diethylaminoazonium dialkoxide and monoclonal antibody, applied in the field of bioengineering, can solve problems such as unclear direct action mechanism and affecting DNA replication of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthetic method and identification of embodiment 1 HL-2:
[0046] (1)
[0047] (2)
[0048]Step a: Slowly add 435 mL of 0.1 M sodium bicarbonate aqueous solution into 80 mL of compound 1 aqueous solution with a concentration of 0.55 mol / L through the dropping funnel. React at room temperature for 20 minutes; pull dry with an oil pump, and then perform final drying on a freeze-drying device to obtain compound 2;
[0049] Step b: Add 12.4g of compound 3 to a 250mL reaction flask, add 100mL of benzene, stir, slowly add 26.6g of NBS, react at room temperature for 5h, TLC detects that the reaction is complete, and compound 4 is obtained;
[0050] Step c: add 0.82g of azobisisobutyronitrile to the reaction flask of compound 4, and add 26.6g of NBS, and reflux at 80°C for 20h; spin off the solvent, then extract with ethyl acetate, wash with saturated brine, no Water Na 2 SO 4 Drying, concentration and column chromatography gave compound 5 as a white solid;
[0051] ...
Embodiment 2
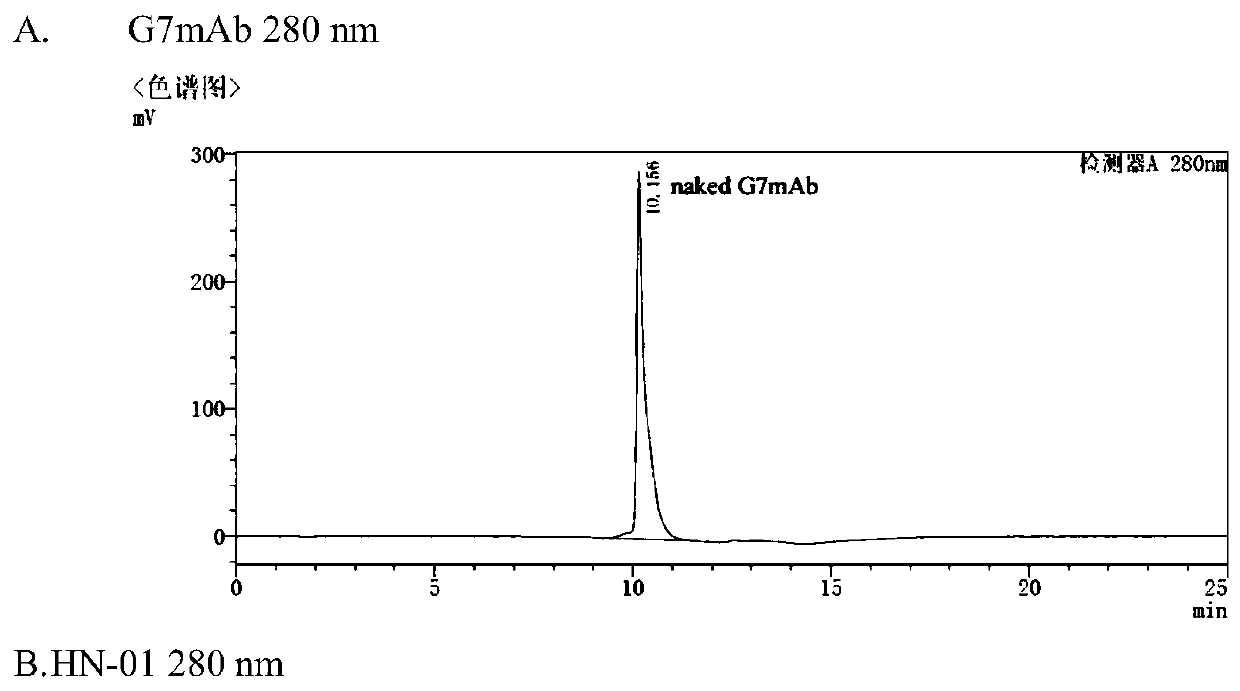
[0066] Example 2 Preparation of Antibody Protein Conjugate HL-01
[0067] 1. Anti-CD24 monoclonal antibody G7mAb (prepared according to literature: Ma Z et al., Selective targeted
[0068] delivery of doxorubicin via conjugating to anti-CD24antibody results in enhanced antitumor potency for hepatocellular carcinoma both in vitro and in vivo.J Cancer Res Clin Oncol.2017,143(10):1929-1940) Purified by Protein A column affinity chromatography and glucose After replacing the antibody solution system with sugar gel G25FF desalting column molecular sieve chromatography, BCA method was used to measure the antibody concentration, and the reducing agent TCEP was mixed with the antibody at a molar ratio of 2.5:1. After adding, stir slowly and evenly, react at 4°C for 1h, 4000rpm, low temperature Residual TCEP was removed by ultrafiltration, and replaced with a PBS (pH 7.0) system containing 1M antioxidant diethylenetriaminepentaacetic acid to prepare for coupling reaction.
[0069] 2. ...
Embodiment 3
[0072]Example 3 Non-reducing SDS-PAGE electrophoresis identification of antibody protein conjugate HN-01
[0073] 1. Sample preparation: Take several EP tubes, add 6 μL of 5×Loading Buffer containing 250 mM Tris-HCl (pH 6.8), 10% SDS, 0.5% bromophenol blue, and 50% glycerol, and add 24 μL of samples to each tube in turn, Mix the solution evenly, put it in a boiling water bath for 5 minutes, centrifuge at 8000 rpm for 3 minutes, and set aside.
[0074] 2. Glue dispensing: Fix the double-layer glass plate to the glue dispensing mold, check for leaks with single distilled water, and configure the lower layer separation glue and the upper layer lamination glue respectively. First add the separating gel to the double-layer glass plate, and immediately flatten it with 1mL absolute ethanol. The separation gel in the lower layer was solidified in about 25 minutes, then the stacking gel was added, and the comb teeth were inserted. After the stacking gel is completely solidified, disa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com