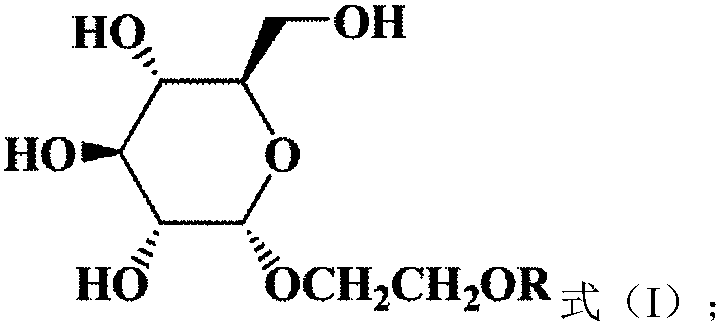
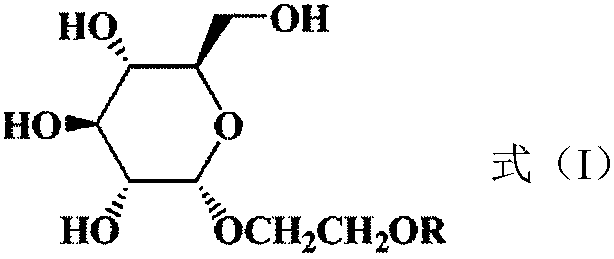
1,2-cis-glucoside surfactant and preparation method thereof
A technology of glucoside and glucopyranoside, which is applied in the field of hydrocarbyloxyethyl glucoside nonionic surfactant and its synthesis, can solve the problems of poor water solubility and easy precipitation of alkyl glucoside, and achieves reduction of solution surface tension, Renewable raw materials and enhanced hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
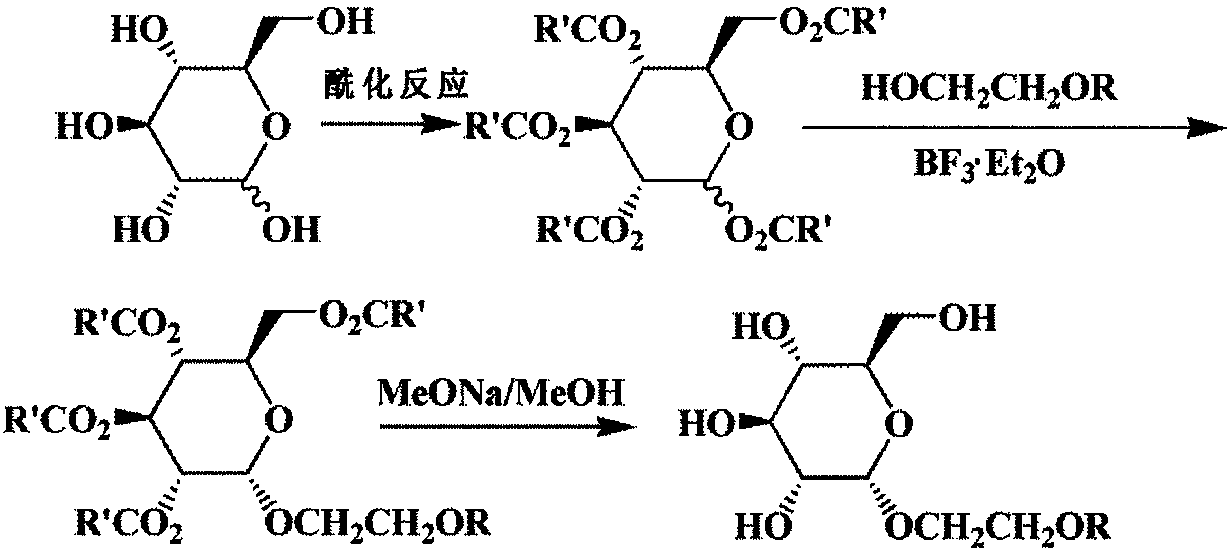
[0028] Add 111.12mmol anhydrous glucose and 944.52mmol acetic anhydride to a 500mL three-neck round bottom flask, heat to 85°C-90°C with stirring, add 50.00mmol anhydrous sodium acetate in batches, heat up to 100°C-110°C, keep warm Reflux reaction, until TLC (V 石油醚 :V 乙酸乙酯 =2:1) The detection reaction is complete. The reaction solution was quickly poured into 600 mL of ice water while it was hot, stirred at room temperature for 12 h, then suction filtered, washed 5 times with 1000 mL of water, and dried. With methanol and water (V 甲醇 :V 水 =1:2.5) mixed solvent for recrystallization to obtain pentaacetylglucose as a white solid with a yield of 86.8%. This product was used directly in reactions in subsequent examples.
Embodiment 1
[0030] Example 1: Hexyloxyethyl-α-D-glucopyranoside
[0031] (1) In a 100mL round bottom flask, add 12.81mmol of pentaacetylglucose and 45mL of Molecular sieve-dried dichloromethane, stirred and dissolved at room temperature, added 12.81 mmol of ethylene glycol monohexyl ether, and added dropwise 57.65 mmol of BF under stirring 3 ·Et 2 O, be warming up to 45 ℃, insulation reaction 16 hours, TLC (V 石油醚 :V 乙酸乙酯 =1.5:1) The detection reaction is complete. The mixed solution was washed successively with saturated aqueous sodium bicarbonate solution and saturated saline solution, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated, and column chromatography (V 石油醚 :V 乙酸乙酯 =3.5:1) separation to obtain hexyloxyethyl-2,3,4,6-tetra-O-acetyl-α-D-glucopyranoside with a yield of 45.3%. used directly in the next reaction.
[0032] (2) Add 11.50 mmol of hexyloxyethyl-2,3,4,6-tetra-O-acetyl-α-D-glucopyranoside and 45 mL of anhydrous methanol to a 100 mL round...
Embodiment 2
[0035] Example 2: Synthesis of Heptyloxyethyl-α-D-Glucopyranoside
[0036] (1) In a 250mL round bottom flask, add 25.62mmol of pentaacetylglucose and 85mL of Molecular sieve-dried dichloromethane, stirred and dissolved at room temperature, added 30.74 mmol of ethylene glycol monoheptyl ether, and added dropwise 115.29 mmol of BF under stirring 3 ·Et 2 O, be warming up to 48 ℃, insulation reaction 16.5 hours, TLC (V 石油醚 :V 乙酸乙酯 =2:1) The detection reaction is complete. The mixed solution was washed successively with saturated aqueous sodium bicarbonate solution and saturated saline solution, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated, and column chromatography (V 石油醚 :V 乙酸乙酯 =4:1) separation to obtain hexyloxyethyl-2,3,4,6-tetra-O-acetyl-α-D-glucopyranoside with a yield of 44.7%. used directly in the next reaction.
[0037] (2) Add 15.00 mmol of the above-prepared heptyloxyethyl-2,3,4,6-tetra-O-acetyl-α-D-glucopyranoside and 55 mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com