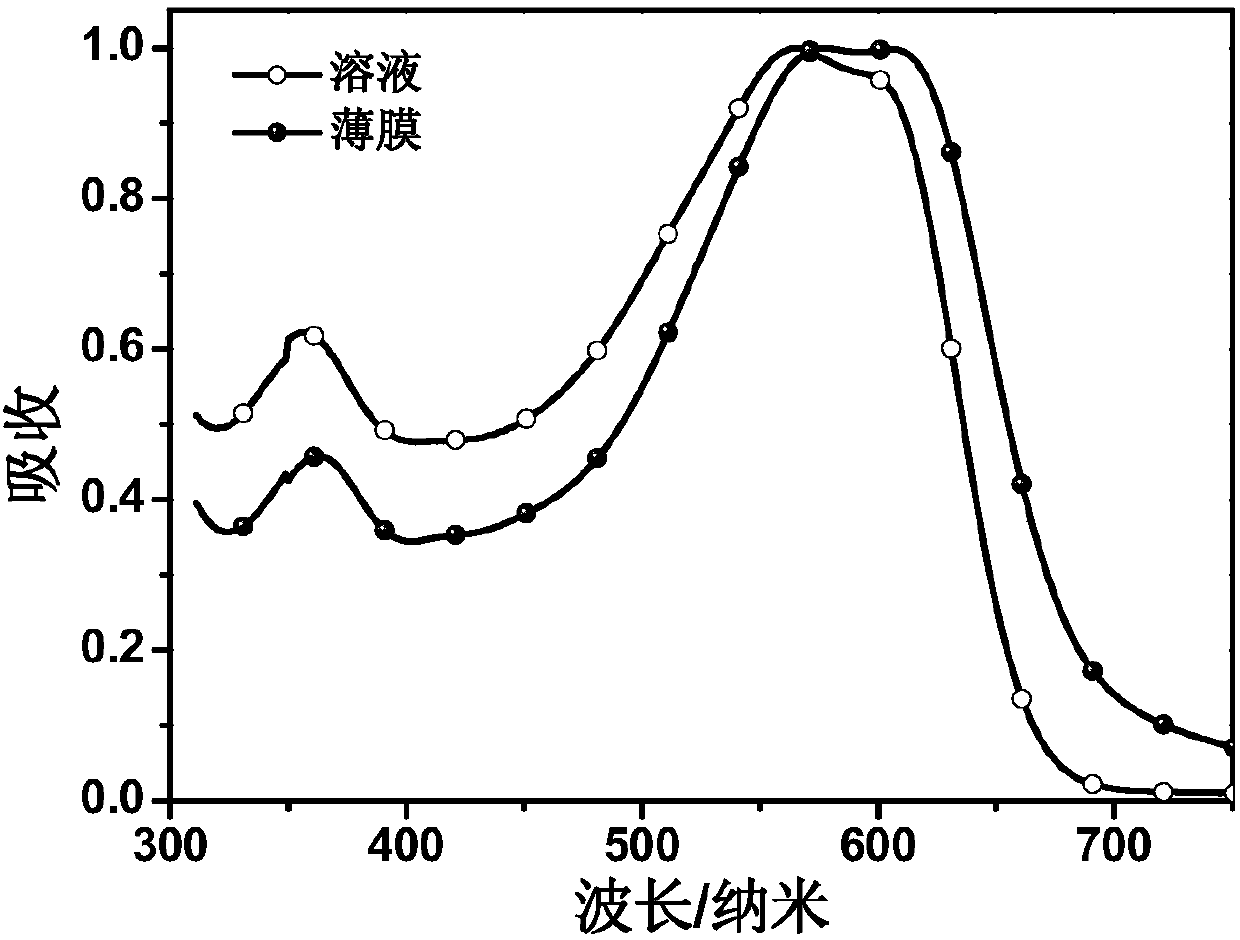
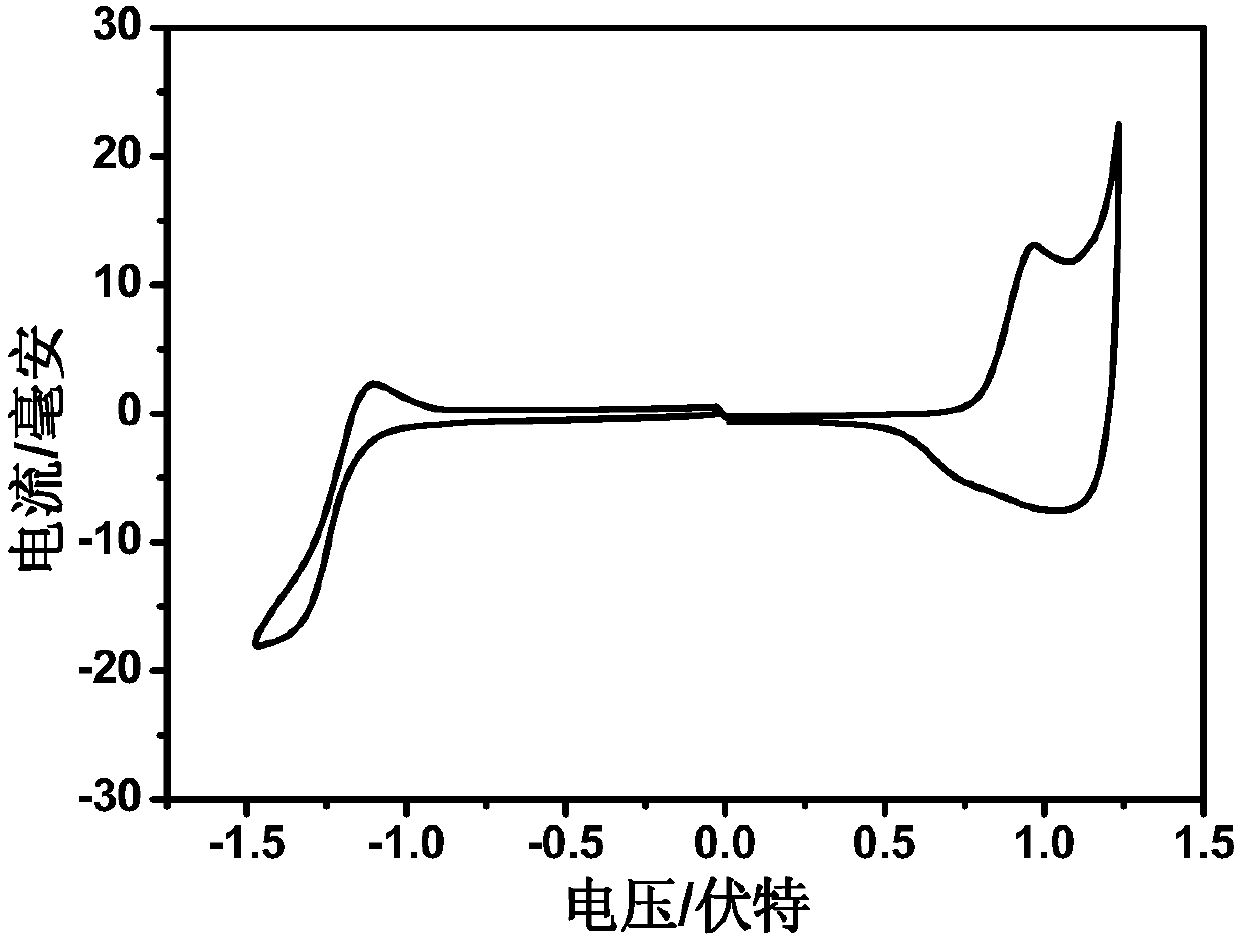
Conjugated polymer containing chlorine-substituted conjugated side chain and preparation method and application thereof
A conjugated polymer, conjugated side chain technology, applied in the molecular field, can solve the problems of expensive raw materials, complicated synthesis, easy aggregation and solubility, etc., and achieves the effects of good light absorption, good thermal stability, and easy synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] The preparation method of the above structure comprises:
[0068] Copolymerize the compound shown in formula II with the compound of formula III with structural formula Y-A-Y in an organic solvent under the action of a catalyst to obtain a conjugated polymer containing chlorine-substituted conjugated side chains shown in formula I;
[0069]
[0070] Among them, Ar 1 、Ar 2 , R 1 , R 2 and the definition of X is the same as formula I;
[0071] X in Formula II 3 Rely on Y in the structure of formula III to select;
[0072] Y in the structure of formula III is selected from any one of boronic acid group, borate ester group or trialkyltin group, X in formula III 3 Any one selected from I or Br; or Y in the structure of formula III is selected from I or Br, X in formula II 3 Any one selected from boronic acid groups, borate ester groups or trialkyltin groups.
[0073] The boronic acid group is selected from but not limited to 1,3,2-dioxaborolan-2-yl, 4,4,5,5-tetram...
Embodiment 1
[0083] This implementation case shows the preparation method of the conjugated polymer containing chlorine-substituted conjugated side chains according to the following steps:
[0084]The reaction scheme is as follows, and the specific reaction steps and reaction conditions are as follows:
[0085]
[0086] (1) Synthesis of compound 2: 3-chlorothiophene (compound 1, 11.9 g, 0.1 mol) and 100 mL of dry tetrahydrofuran (THF) were added into a two-neck round-bottomed flask protected by argon. 50 mL of LDA (lithium diisopropylamide, 0.1 mol, 2.0 M) was slowly added dropwise at -78°C. After stirring was continued for 2 hours at -78°C, isooctane bromide (20.4 g, 0.11 mol) was added. After continuing the reaction at -78°C for 0.5 hours, the reaction was stirred overnight at room temperature, then quenched with 50 mL of water, and the mixture was extracted twice with diethyl ether. After removing the organic solvent, the crude product was separated by silica gel (200-300 mesh) col...
Embodiment 2
[0098] Preparation and performance testing of organic solar cell devices:
[0099] Commercially purchased indium tin oxide (ITO) glass was scrubbed with acetone first, then washed with detergent, water, deionized water, acetone, and isopropanol in sequence, and after drying, spin-coated a layer of 30nm-thick zinc oxide as the cathode The modified layer was dried at 200°C for 60 minutes and set aside. Spin-coat the toluene blend solution (10-30mg / ml) of the conjugated polymer containing chlorine-substituted conjugated side chains and the small molecule electron acceptor material IT-4F (weight ratio: 1.25:1) in the examples The active layer of the device is formed on the zinc oxide cathode modification layer. Then, the active layer was thermally annealed at 180° C. for 10 minutes under a nitrogen atmosphere. Finally, at about 10 -4 Sequential evaporation of MoO under pressure of Pa 3 (10nm) as the anode modification layer and Al (80nm) as the anode of the device to obtain a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com