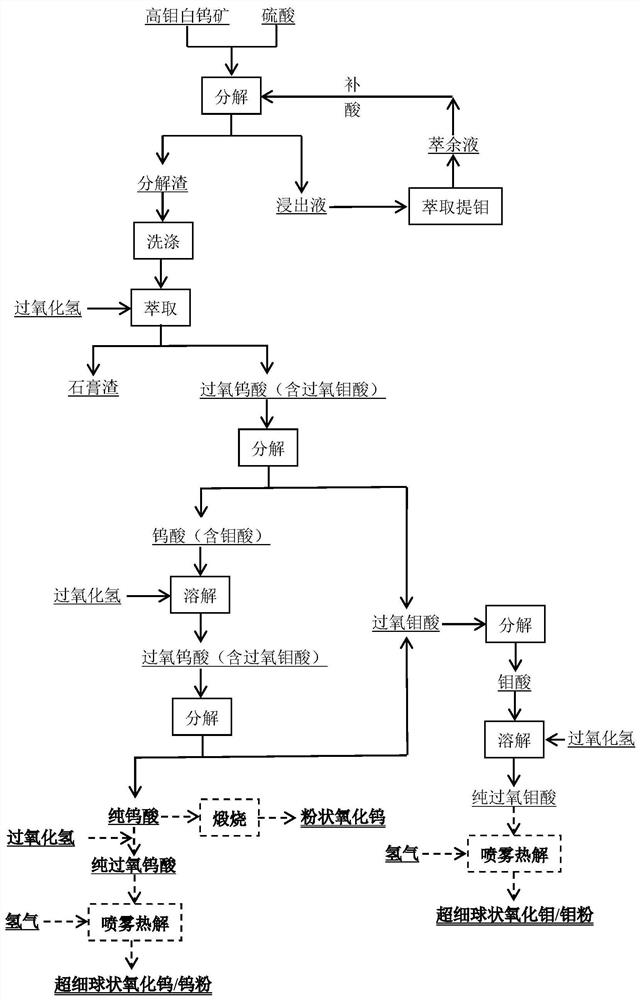
A method for extracting tungsten and molybdenum from high-molybdenum scheelite
A technology of scheelite, tungsten and molybdenum, applied in the direction of improving process efficiency, etc., can solve problems such as high price, environmental pollution, hydrogen sulfide harmful production and environment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Raw material of high molybdenum scheelite used in this embodiment: WO 3 The grade is 40%, the Mo grade is 5%, and the particle size is 120 μm.
[0092] A method for extracting tungsten and molybdenum from high-molybdenum scheelite, comprising the steps of:
[0093] (1) Leaching reaction: Add high-molybdenum scheelite into a sulfuric acid solution with a concentration of 150g / L. The liquid-solid ratio of the system is 5:1. The leaching reaction is carried out at 150°C and 0.5Mpa for 3 hours; 99.5% Tungsten is converted into tungstic acid, 10.5% of molybdenum is converted into molybdenum acid, and the leaching rate of molybdenum is 89.1%;
[0094] (2) extracting molybdenum in the leachate: filter the reaction product obtained in step (1) to obtain decomposition residue and leachate; add extractant (30% N235+10%TBP+kerosene) to the gained leachate to control the volume of the organic phase and the water phase The ratio is 1:1, mixed, and extracted at 40°C for 10 minutes ...
Embodiment 2
[0102] Raw material of high molybdenum scheelite used in this embodiment: WO 3 The grade is 30%, the Mo grade is 25%, and the particle size is 50 μm.
[0103] A method for extracting tungsten and molybdenum from high-molybdenum scheelite, comprising the steps of:
[0104] (1) Leaching reaction: Add high-molybdenum scheelite into a sulfuric acid solution with a concentration of 300g / L, and at the same time add calcium sulfate with 5% tungsten ore mass. The liquid-solid ratio of the system is 5:1. At 200°C, 1.85 The leaching reaction was carried out under the condition of Mpa for 3 hours; 99.5% of tungsten was converted into tungstic acid, 8.5% of molybdenum was converted into molybdenum acid, and the leaching rate of molybdenum was 91.2%;
[0105] (2) Extract molybdenum in the leachate: filter the reaction product obtained in step (1) to obtain decomposition slag and leachate; add extractant (40% P204+kerosene) to the gained leachate, and control the organic phase to water pha...
Embodiment 3
[0113] Raw material of high molybdenum scheelite used in this embodiment: WO 3 The grade is 55%, the Mo grade is 2.5%, and the particle size is 150 μm.
[0114] A method for extracting tungsten and molybdenum from high-molybdenum scheelite, comprising the steps of:
[0115] (1) Leaching reaction: Add high-molybdenum scheelite into sulfuric acid solution with a concentration of 200g / L, and at the same time add tungstic acid with 10% tungsten ore mass. The liquid-solid ratio of the system is 7:1. Carry out leaching reaction for 4 hours; 99.3% of tungsten is converted into tungstic acid, 1.2% of molybdenum is converted into molybdenum acid, and the leaching rate of molybdenum is 98.2%;
[0116] (2) Molybdenum extraction in the leachate: filter the reaction product gained in step (1) to obtain decomposition slag and leachate; add extractant (35% LIX63+kerosene) to the gained leachate, control the volume ratio of the organic phase to the water phase to be 1: 1. Mix, carry out mol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com