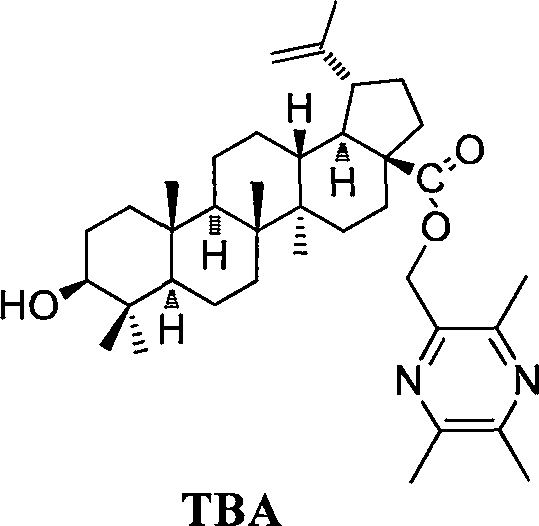
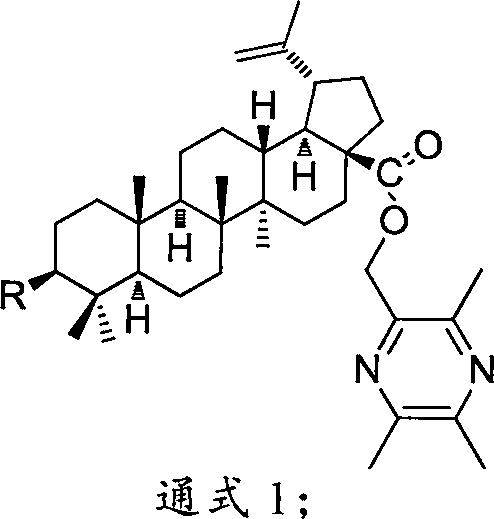
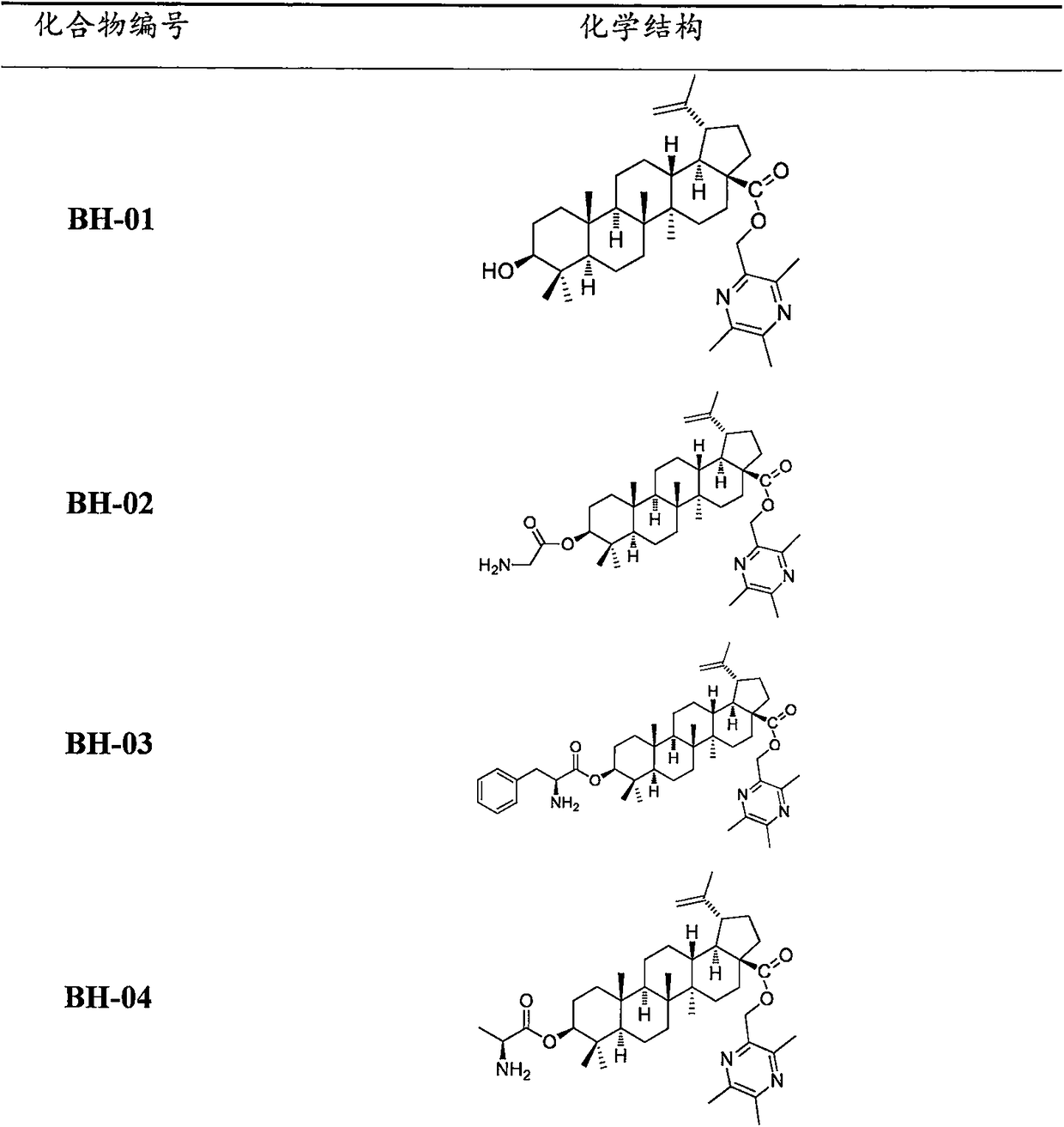
Compound BA-X having antitumor effect, preparation method and applications thereof
A technology of anti-tumor effects and compounds, applied in anti-tumor drugs, steroids, organic chemistry, etc., can solve the problems of lack of selectivity and side effects of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1 Preparation of intermediate compound 1 (2-chloromethyl-3,5,6-trimethylpyrazine)
[0101] Take Ligustrazine 2.176g (16mmol) and dissolve it in 20ml glacial acetic acid, add 1.8ml (16mmol) 30% hydrogen peroxide and react at 90°C for 4h, then add 1.8ml (16mmol) 30% hydrogen peroxide to continue the reaction for 2h, and monitor the reaction by TLC Completely, add an appropriate amount of sodium sulfite to neutralize excess hydrogen peroxide, filter the reaction solution, cool the filtrate to room temperature, adjust the pH to 10 with 50% sodium hydroxide, extract with dichloromethane, collect the organic layer, and dehydrate with saturated saline. Dry over sodium sulfate, and recover the solvent under reduced pressure to obtain the crude white ligustrazine nitrogen oxide compound (2). Add 1.51ml (16mmol) of acetic anhydride to the crude product, heat to reflux at 105°C for 2.5h, monitor by TLC until the reaction is complete, then evaporate to dryness under reduced...
Embodiment 3
[0102] The preparation of embodiment 3 BH-01
[0103] Weigh 2.28g (5mmol) betulinic acid, 0.69g (5mmol) anhydrous potassium carbonate in a 50ml reaction bottle, add 25mlDMF, mix for half an hour, add 1.02g (6mmol) ligustrazine chloride, under nitrogen protection, at room temperature Under reaction for 12h, TLC detects that the reaction is complete, and a certain amount of water is added to terminate the reaction, followed by extraction with dichloromethane, and the organic layer is collected, and an appropriate amount of anhydrous sodium sulfate is used to remove water, evaporated to dryness under reduced pressure, and separated on a silica gel column [V (petroleum ether ): V (acetone) = 10: 1] to obtain 2.66 g of white solid, yield 95%. mp: 184.6-185.4°C, [α] D =+16(c 0.50, MeOH); 1 H-NMR (CDCl 3 )(ppm): 0.78, 0.80, 0.82, 0.96, 0.98, 1.67(s, 3H, 30-CH 3 of BA), 2.51(s, 3H, -CH 3 ), 2.53(s, 3H, -CH 3 ), 2.57(s, 3H, -CH 3 ), 3.02(m, 1H), 3.19(m, 1H), 4.61, 4.74(each, brs...
Embodiment 4
[0104] Example 4 Preparation of Compound BH-02
[0105] Sequentially weigh 200.00mg (0.338mmol) TBA, 77.04mg (0.44mmol) Boc-L-glycine, 97.20mg (0.50mmol) EDCI and 4.12mg (0.038mmol) DMAP into a reaction bottle containing 5ml of anhydrous DCM , stirred overnight at room temperature, when TLC detected that TBA was basically consumed, the reaction solution was diluted with 20ml of dichloromethane and washed with appropriate amount of water and saturated NaCl solution successively, dehydrated with anhydrous sodium sulfate, concentrated under reduced pressure and then separated on a silica gel column [V(2 Chloromethane): V (methanol) = 40: 1] to obtain a white solid; place the white solid in an ethyl acetate solution containing 3M HCl under ice bath and stir for 1 h, concentrate under reduced pressure to remove most of the HCl gas, and then add an appropriate amount of saturated NaHCO 3 Adjust the pH of the aqueous solution to about 8-9, extract with dichloromethane, dehydrate the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com