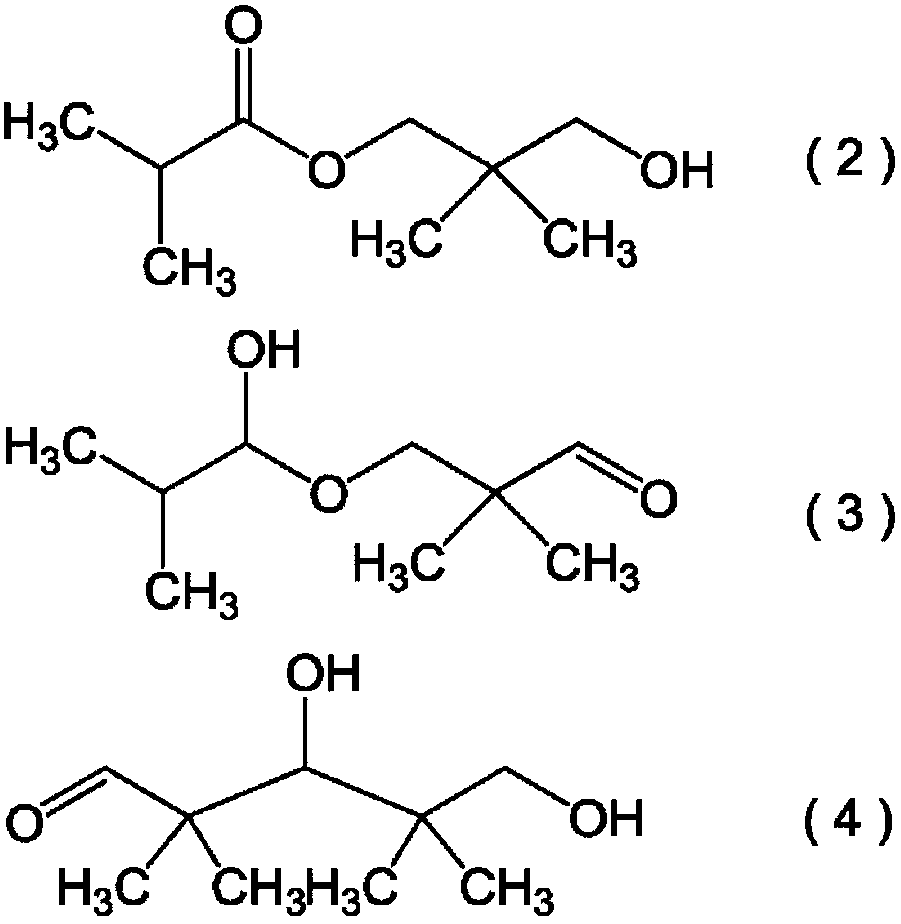
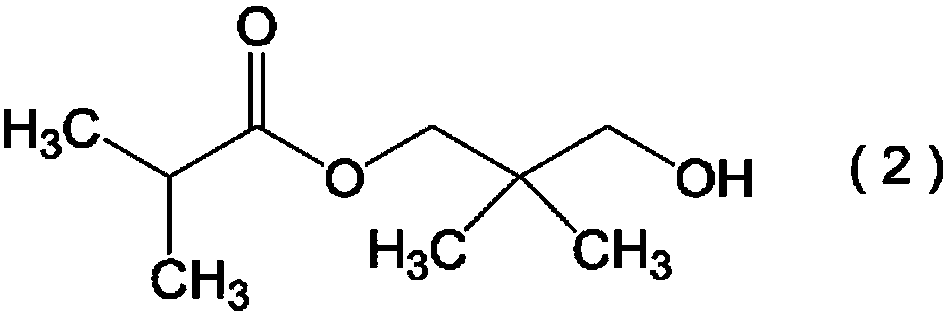
Hydroxypivalaldehyde production method
A technology of hydroxypivalaldehyde and its manufacturing method, which is applied to the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds. It can solve problems such as hindering hydrogenation and affecting the quality of SPG, and achieve the effect of improving the recovery rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0174] Hereinafter, although an Example demonstrates this invention concretely, this invention is not limited to these Examples at all. However, in the following examples and comparative examples, unless otherwise specified, "parts" and "%" mean "parts by mass" and "% by mass", respectively.
[0175]Among them, in the examples, formaldehyde was quantified by absorbance measurement by the acetylacetone method, formate was quantified by capillary electrophoresis, and other components were quantified by gas chromatography. In addition, the amount of water in the extract oil layer was measured using a Karl-Fischer moisture meter (manufactured by Hiranuma Sangyo Co., Ltd., automatic moisture measuring device AQV-2200, and the titrant used HYDRANAL-Composite 5K manufactured by Hayashi Junyaku Kogyo Co., Ltd.) . Conditions for absorbance measurement, capillary electrophoresis, and gas chromatography are shown below.
[0176] [Measurement method and conditions]
[0177]
[0178] ...
reference example 1
[0203] Add 464g of IBAL (6.43mol) and 438g (5.83mol) of 40% formalin in a 2L three-necked flask with a stirring device and a cooler, and add 28g (0.277mol) of triethylamine as a catalyst while stirring , continue stirring at 90°C for 2 hours to synthesize HPA. After stirring, the HPA reaction liquid was returned to room temperature, and quantitative analysis of components was performed. Table 1 shows the composition of components in 930 g of the obtained HPA reaction liquid.
[0204] [Table 1]
[0205]
[0206]
[0207] Add 606.7 g of the HPA reaction solution obtained by the same method as Reference Example 1, 601.1 g of IBAL, and 401.93 g of pure water into a three-necked flask with a capacity of 2 L having a stirring device and a cooler, adjust the liquid temperature to 60° C., and stir 60 minutes. After you stop stirring, let it stand for about 10 minutes to allow it to separate into two layers. The upper layer was recovered to obtain 1017.5 g of an extract (oil l...
Embodiment 1
[0234] [Example 1: Continuous distillation]
[0235] The extract obtained by the same operation as in Reference Example 1 was mixed with water at a flow rate of 2.5 g / min and a flow rate of 3.0 g / min. There is steam with an absolute pressure of 0.6MPa in the pipe), and when the liquid is passed, the absolute pressure is 0.33MPa, and the residence time in the preheater is 10 minutes, and it is supplied to the The Dixon packing (Dixon packing) distillation column has a tower height of 500 mm, and the distillation tower has a tower diameter of 30 mm and a tower height of 800 mm. Here, the content of IBAL in the extract was 51.2% by mass, and the flow rate of IBAL was 1.28 g / min (0.512×2.5 g / min=1.28 g / min). In addition, the water content in the extract was 6.2% by mass, and the flow rate of water supplied from the extract was 0.155 g / min (0.062×2.5 g / min≒0.16 g / min). Therefore, the amount of water supplied for distillation is 247 parts by mass ((3.0+0.16)÷1.28×100≒247 with resp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com