Nobiletin antigen as well as preparation method and application thereof
A technology of nobiletin and antigen, which is applied in the field of nobiletin antigen and its preparation, which can solve the problems of high detection cost, inapplicability of rapid detection, and long time consumption, and achieve low synthesis cost, simple and clear synthesis steps, and good effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
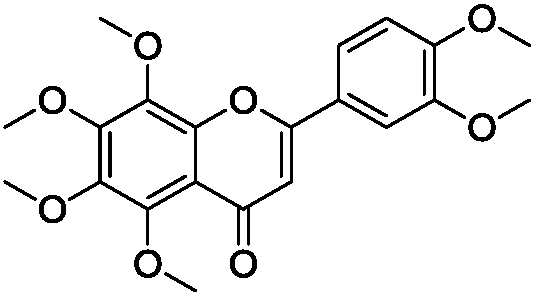
[0044] Embodiment 1, the preparation of nobiletin-ovalbumin (nobiletin-OVA) antigen
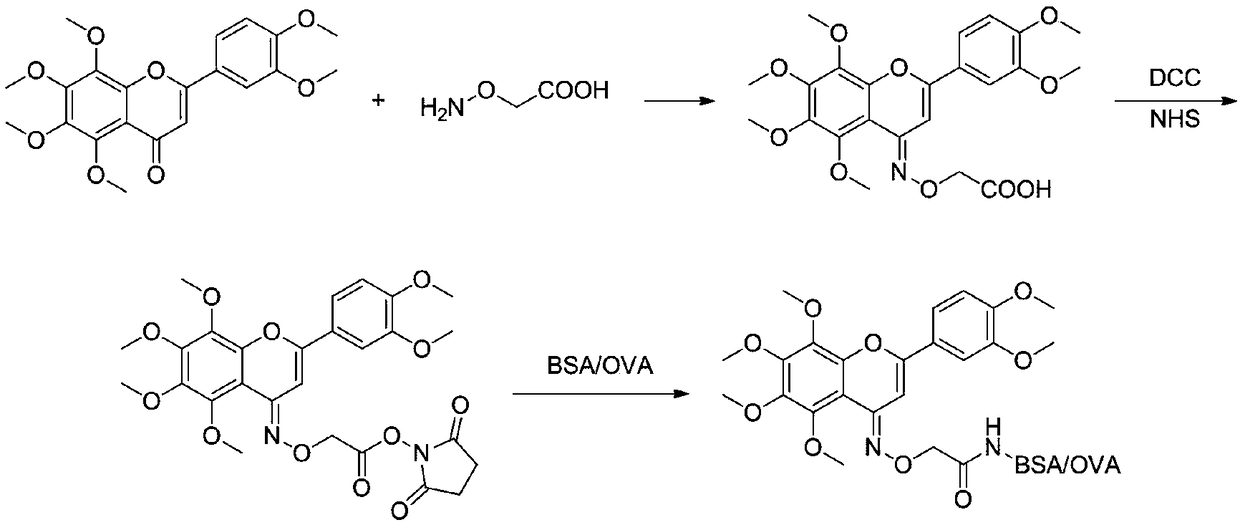
[0045] Synthetic roadmap as figure 1 shown.
[0046] 1) Synthesis of compound shown in formula I
[0047] In a 50mL three-necked flask, add 0.3g nobiletin and 0.2g carboxymethoxylamine hemihydrochloride, then add 15mL pyridine, heat to 100°C, react for 24h and cool to room temperature, pour the reaction solution into 100mL water, Adjust the pH value to 3 with concentrated hydrochloric acid, extract with ethyl acetate (3×50 mL), dry over anhydrous sodium sulfate, spin precipitation, and column chromatography to obtain 0.2 g of the product with a yield of 56%.
[0048] 1 H NMR(600MHz,dmso)δ12.70(s,1H),7.48(d,J=8.3Hz,1H),7.36(s,1H),7.09(d,J=8.5Hz,1H),7.05(s ,1H),4.63(s,2H),3.93(s,3H),3.89(s,3H),3.84(s,3H),3.81(s,3H),3.79(s,3H),3.63(s, 3H).
[0049] 13 C NMR(151MHz,dmso)δ171.97,153.34,151.40,149.28,148.67,146.58,144.52,144.11,142.98,138.31,124.42,119.01,112.23,108.82,108.70,92.01,70.86,62....
Embodiment 2
[0061] Embodiment 2, the preparation of nobiletin-bovine serum albumin (nobiletin-BSA) antigen 1) the synthesis of nobiletin hapten shown in formula I and its activation are no different from those in Example 1, no longer repeat.
[0062] 2) Slowly drop the compound shown in the formula II obtained in step 2) in Example 1 into the carrier protein solution
[0063] (The carrier protein solution is 157.5mg BSA dissolved in 10mL phosphate buffered saline (PBS) with a pH value of 7.4), the molar ratio of the compound of formula II to the carrier protein is 15:1, and stirred overnight at 4°C.
[0064] 3) Dialysis: The reaction solution obtained in step 2) was dialyzed for three days with a PBS solution with a pH value of 7.4 and a concentration of 0.01mol / L, and the completely dialyzed reaction product solution (Nobiletin-BSA) was diluted to 1 mg / mL The solution was frozen at -40°C until use. The function of dialysis is to remove unreacted nobiletin haptens and other small molecu...
Embodiment 3
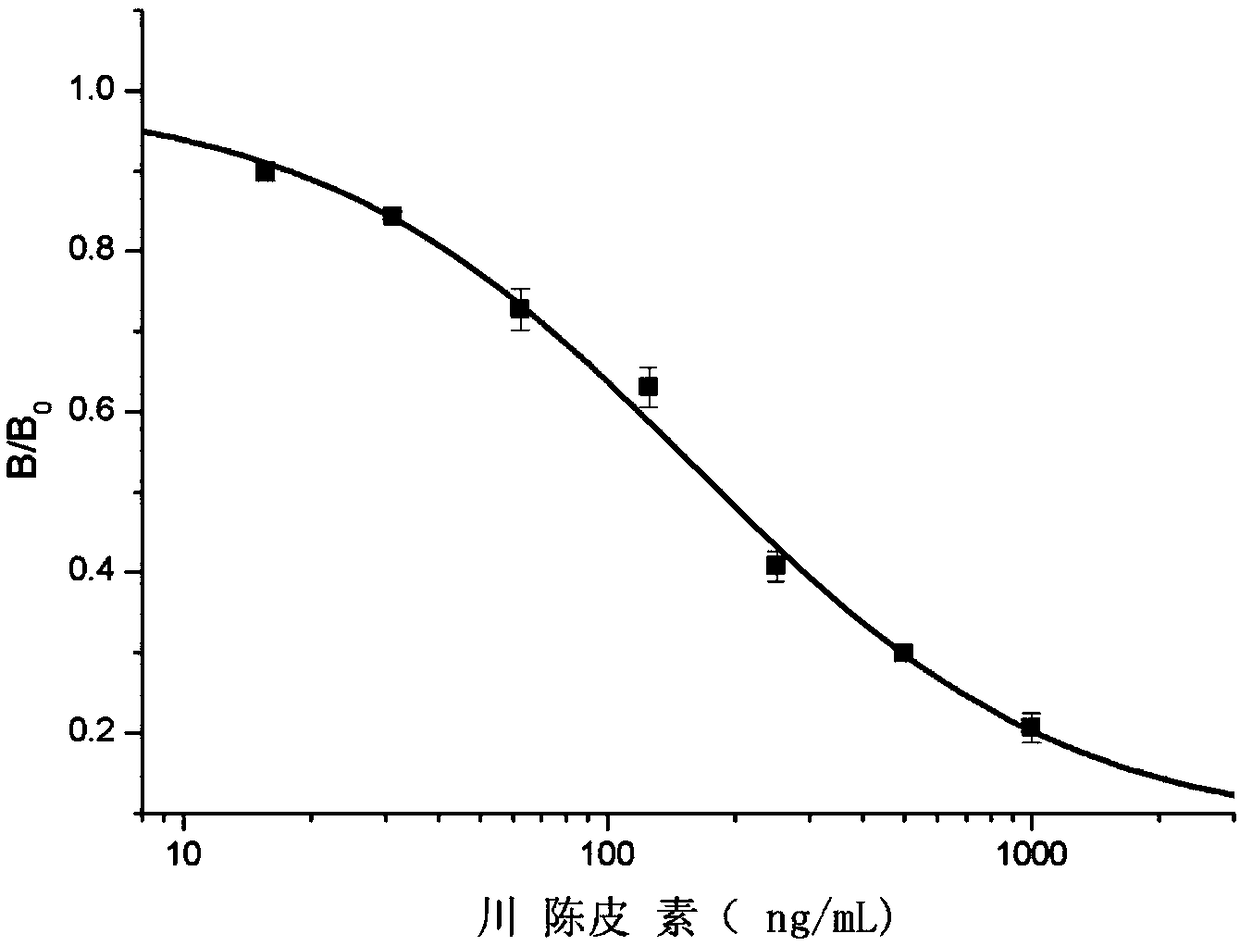
[0067] Embodiment 3, application of nobiletin-bovine serum albumin (nobiletin-BSA) antigen
[0068] 1. Preparation of Antibody Using Nobiletin-Bovine Serum Albumin (Nobiletin-BSA) Antigen
[0069] (1) Take 8-10 week old Bal b / c mice as experimental animals.
[0070] (2) Basic immunization: obtain the diluted nobiletin-BSA antigen solution (concentration is 1mg / mL) from the embodiment 2, add an equal volume of Freund's complete adjuvant after filtering through a sterile filter, and use a magnetic stirrer to Stir well to emulsify until dripping into water does not spread. Bal b / c mice were injected subcutaneously with emulsified complete antigen at multiple points in the abdominal cavity and back, and the injection dose was 0.1 mg emulsified antigen / mouse.
[0071] (3) Booster immunization: 2 weeks after the basic immunization, take 1 mL of the above-mentioned diluted nobiletin-BSA antigen solution, then add 1 mL of Freund's incomplete adjuvant, fully stir and emulsify with a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com