Mussel attachment protein/amphoteric ion polypeptide fusion protein with attachment-anti-staining dual functions and synthesis method
A technology of mussel adhesion protein and fusion protein, which is applied in the directions of fusion polypeptides, chemical instruments and methods, antibody mimics/scaffolds, etc., can solve the problems of high cost and low output, and achieves low cost, low equipment requirements, Anti-fouling effect is obvious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1: Construction of recombinant expression vector and bacterial strain
[0067] ①Construction of recombinant expression vector
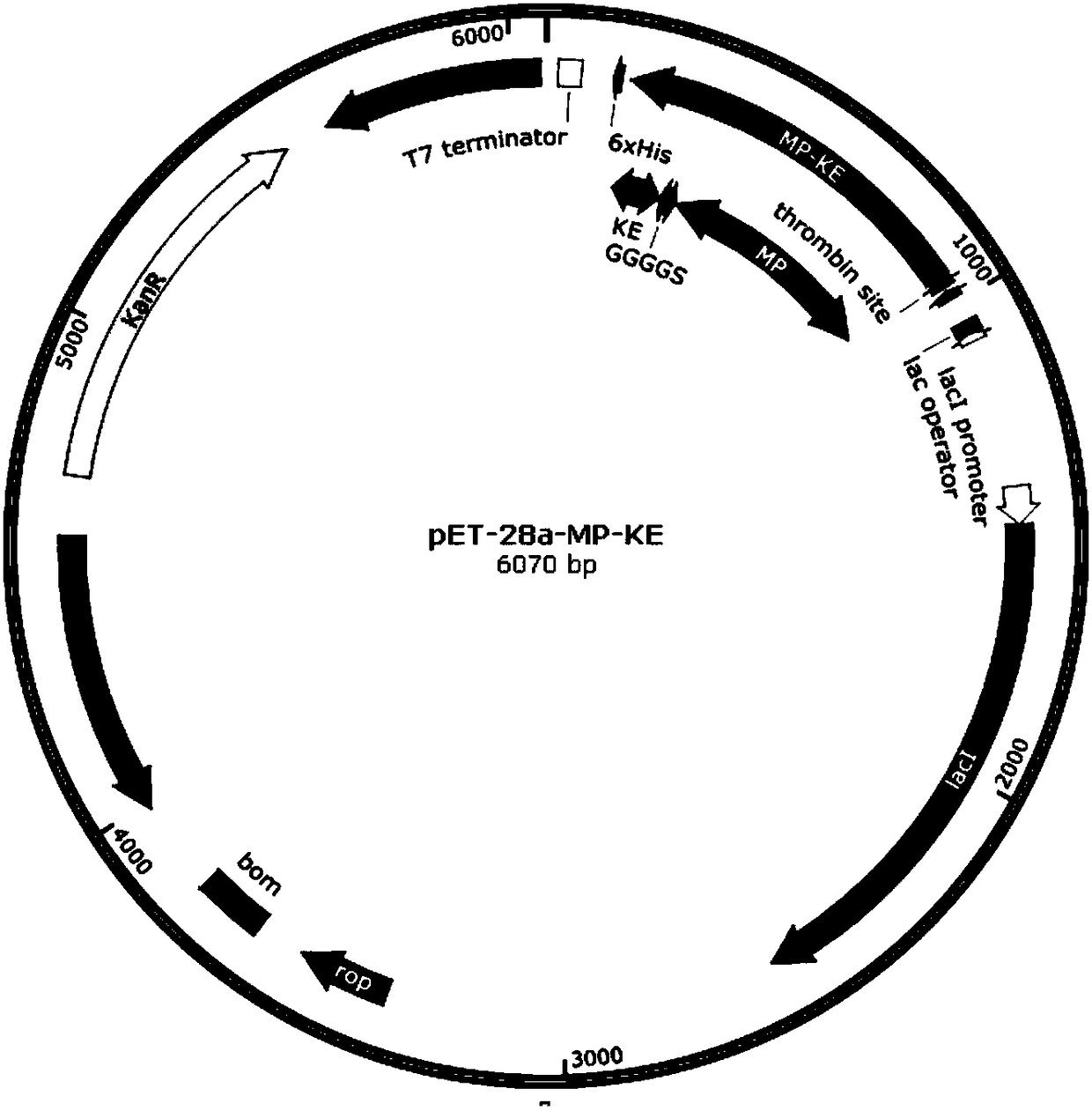
[0068] Using 3 GGGGS repeat sequences as connecting polypeptides to connect fusion protein MP and 20 KE repeat sequences of zwitterionic polypeptides to form fusion protein MP-KE, the gene is coded according to the expression preference of Escherichia coli without changing its amino acid sequence sub-optimization. A NdeI restriction site was added at the 5' end of the gene sequence and a HindIII restriction site was added at the 3' end of the gene. The gene sequence was artificially synthesized and cloned into the Escherichia coli expression vector pET-28a to obtain the recombinant expression vector pET-28a-MP-KE, which contained T7 strong promoter, lca lactose operon, kanamycin-resistant Sex tagging sites and hexahistidine tags, such as figure 1 shown.
[0069] ②Chemical transformation of Escherichia coli
[0070] Thaw competen...
Embodiment 2
[0071] Embodiment 2: Expression and purification of fusion protein MP-KE
[0072] ① Fermentative expression of fusion protein MP-KE in Escherichia coli
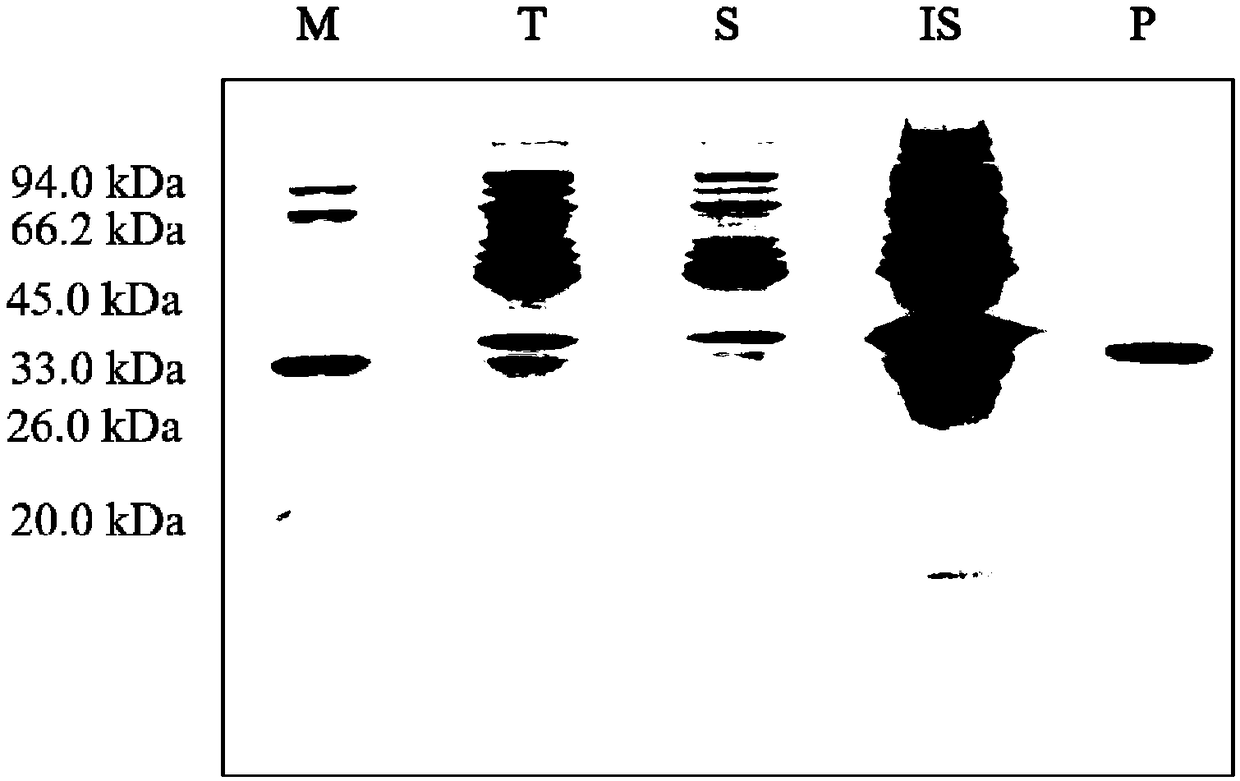
[0073] The successfully transformed Escherichia coli was streaked and activated on a double-resistant LB solid medium plate containing kanamycin and chloramphenicol. A single colony was picked and placed in a 5 ml test tube containing 50 μg / ml kanamycin and chloramphenicol LB liquid medium, and cultured overnight at 37° C. with shaking at 200 rpm. Transfer the E. coli seed solution to a 500ml shake flask containing 200ml medium at a ratio of 1:100. Measure the growth curve of Escherichia coli with a UV spectrophotometer. After the E. coli is transferred, when the concentration of the bacterial solution is OD600=0.8, use 0.8mM IPTG to induce E. coli to express the fusion protein MP-KE, at 37°C, under the condition of 250rpm After continuing to cultivate for 12 h, E. coli cells were collected.
[0074] ②Isolation and purific...
Embodiment 3
[0082] Example 3: In vitro hydroxylation and DOPA validation
[0083] ①In vitro hydroxylation reaction
[0084] The 0.1MPBS system containing 1mg / ml fusion protein MP-KE, 250U / ml tyrosinase, 20mM sodium borate, 25mM ascorbic acid was stirred at 25°C and bubbled for 5h to hydroxylate the tyrosine residue in the fusion protein to DOPA . Real-time control of reaction temperature and bubbling speed during the reaction process. After the reaction, the solution was dialyzed, ultrafiltered and freeze-dried.
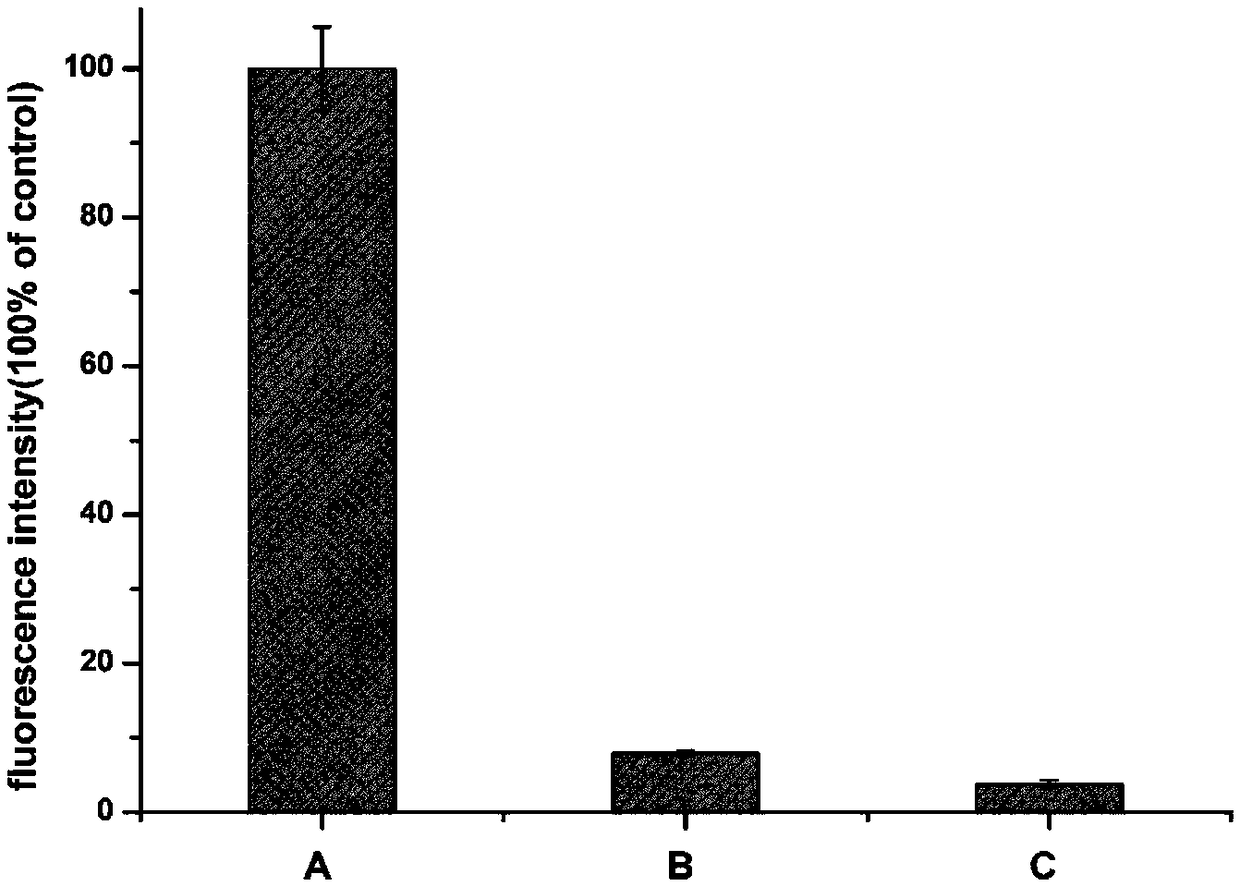
[0085] ②Nitroblue tetrazolium (NBT) staining
[0086] Three sample solutions of water, fusion protein MP-KE and modified fusion protein were respectively dropped on the polyvinylidene fluoride (PVDF) membrane. After the protein samples were bound to the membrane, soak the PVDF membrane in 2M potassium glycinate (pH 10) solution containing 0.5mg / ml nitroblue tetrazolium (NBT) and treat it in the dark for 1h. The PVDF membrane was washed sequentially with 0.2M sodium borate (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com