Preparation process of alpha-damascenone
A preparation process, damascenone technology, applied in the field of α-damascenone preparation process, can solve the problems of expensive reagent yield, difficult to achieve mass production, strict conditions, etc., to avoid high operating costs, experimental Conditions and subsequent processing are easy and the effect of low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044]The preparation process of a kind of α-damascenone described in the present invention uses α-ionone, namely compound A, as a raw material, and reacts with hydroxylamine hydrochloride to obtain α-ionone oxime, namely compound B, and the double bond in compound B is oxidized After dehydration, α-ionone epoxidized oxime is compound C, and compound C is dehydrated under the action of acid to obtain α-ionone isoxazole derivative, which is compound D, and compound D is reduced to obtain the final product E, which is α-da Matone;
[0045] The structural formula of compound A:
[0046]
[0047] The structural formula of compound B:
[0048]
[0049] The structural formula of compound C:
[0050]
[0051] The structural formula of compound D:
[0052]
[0053] The structural formula of the final product E:
[0054]
[0055] Its reaction formula is as follows:
[0056]
[0057] The preparation technology of described a kind of α-damascenone, specifically com...
Embodiment 1
[0075] [Example 1] Synthesis of α-ionone oxime, compound B;
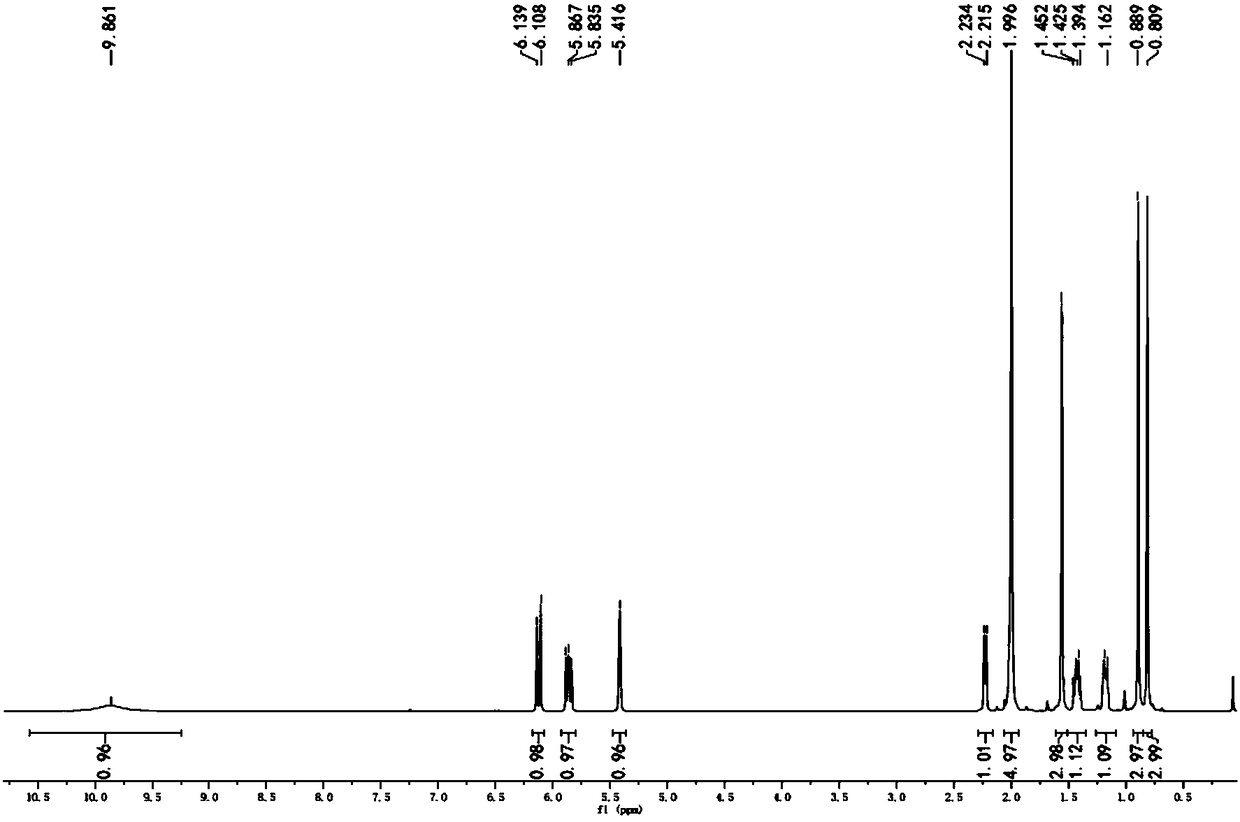
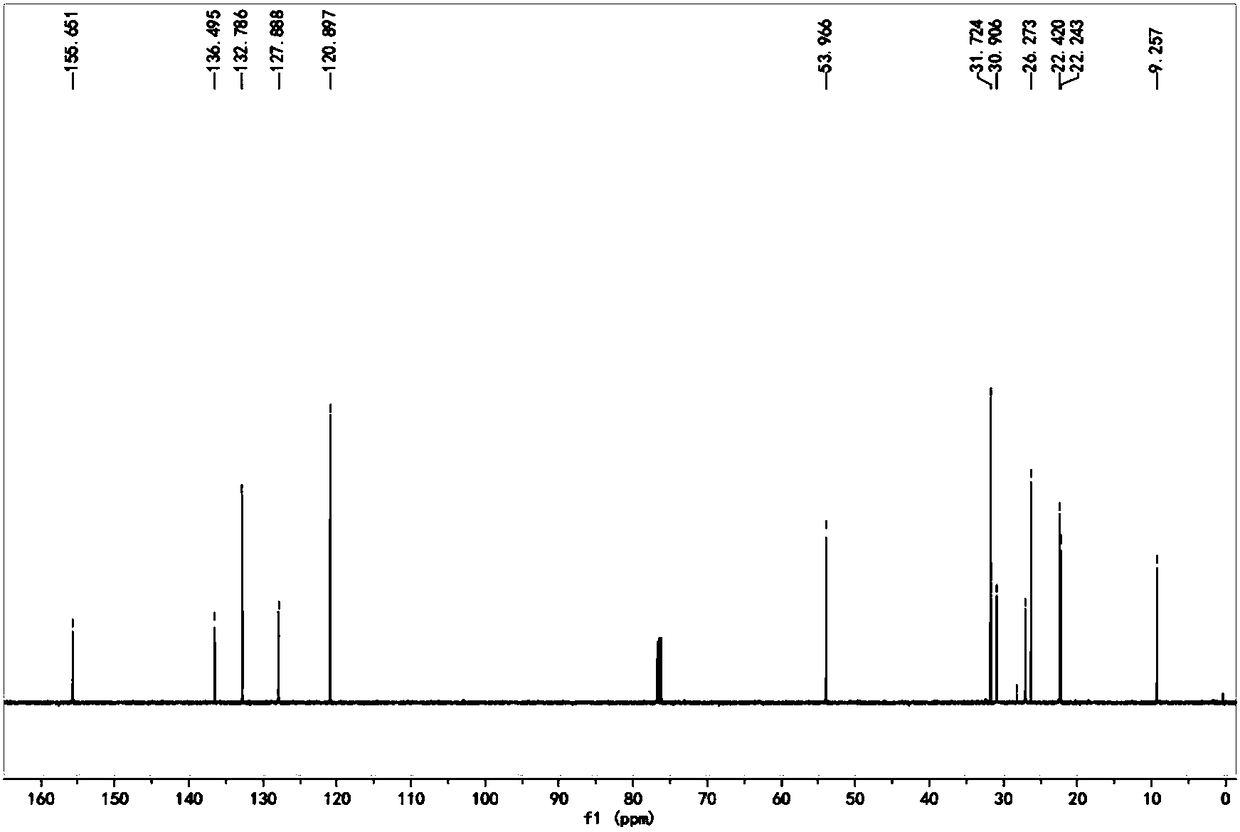
[0076] Take 10g, 0.052mol of α-ionone, that is, compound A, dissolve it with 20ml of ethanol, add it to the flask, add 3.8g, 0.06mol of hydroxylamine hydrochloride, 6.4g, 0.07mol of sodium acetate and 15ml of ionized water. After all the addition, the temperature of the system was raised to 55° C., and the reaction time was controlled for 3 hours. After the reaction, add 60ml of deionized water to the system, extract with ethyl acetate, combine the organic phases, and wash the organic phases with 10% saturated sodium bisulfite solution, anhydrous MgSO 4 After drying and rotary evaporation, an orange-yellow liquid was obtained, which was solid in a refrigerator, that is, α-ionone oxime, that is, compound B, and the yield was 99.6%.
[0077] The H NMR spectrum of α-ionone oxime, namely compound B, is as follows figure 1 Shown; α-ionone oxime is the carbon NMR spectrogram of compound B as figure 2 shown.
Embodiment 2
[0078] [Example 2] Synthesis of α-ionone epoxidized oxime, compound C;
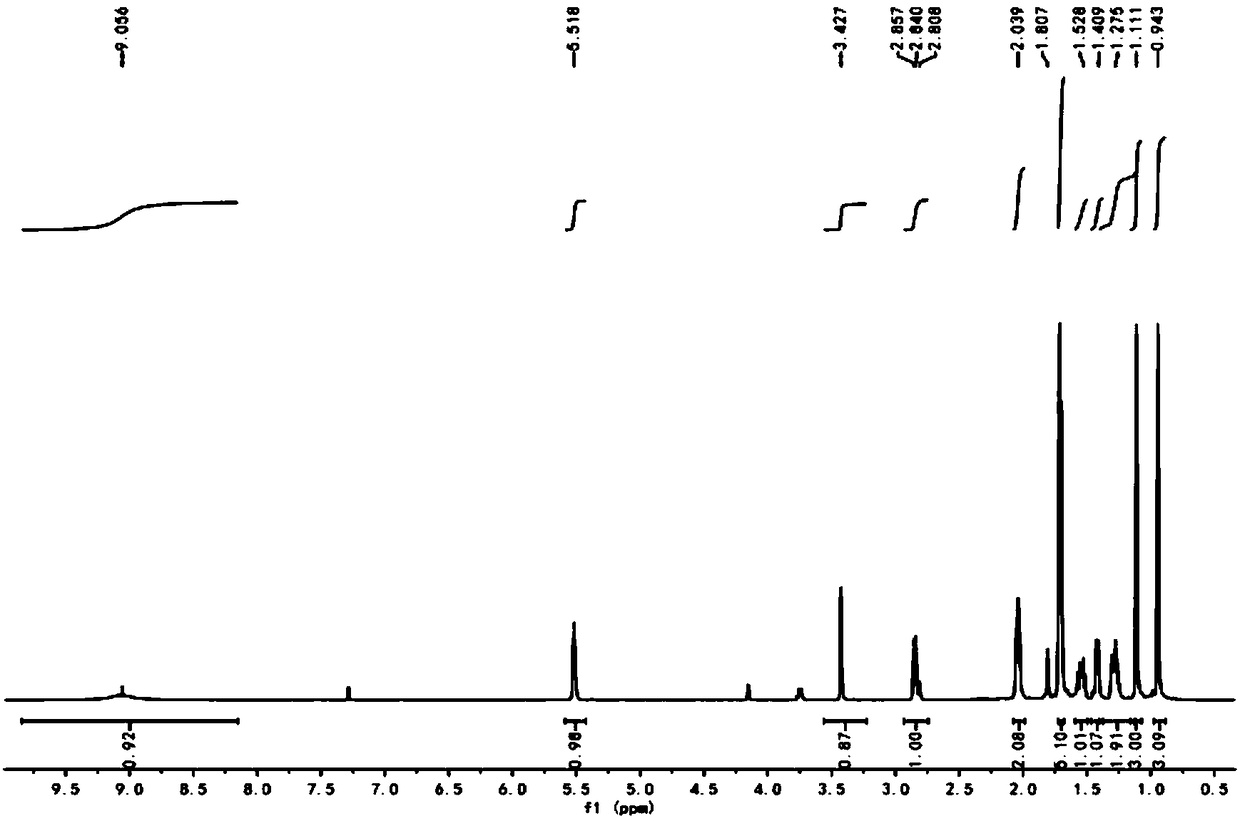
[0079] After mixing 5 g and 0.024 mol of α-ionone oxime, that is, compound B, with 70 ml of methanol, they were added to the flask. Under stirring conditions, slowly add 35ml of 30% hydrogen peroxide and 5ml, 6mol / L lithium hydroxide mixture, after the addition, weigh 70mg of potassium carbonate, slowly add into the reaction system, maintain the system temperature at 25°C, and the reaction time is 15h . After the reaction is complete, add 150ml deionized water, extract with dichloromethane, combine the organic phases, and use anhydrous MgSO 4 After drying and rotary evaporation, the α-ionone epoxidized oxime, compound C, was obtained with a yield of 99.1%.
[0080] The H NMR spectrum of α-ionone epoxidized oxime, compound C, is as follows: image 3 Shown; α-ionone epoxidized oxime is the NMR carbon spectrogram of compound C as Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com