A kind of preparation method of bupropion hydrochloride
A technology of bupropion hydrochloride and bupropion hydrochloride is applied in the field of preparation of bupropion hydrochloride, can solve the problems of not taking into account, restricting industrial production, not completely solving bromine pollution and the like, and achieving the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
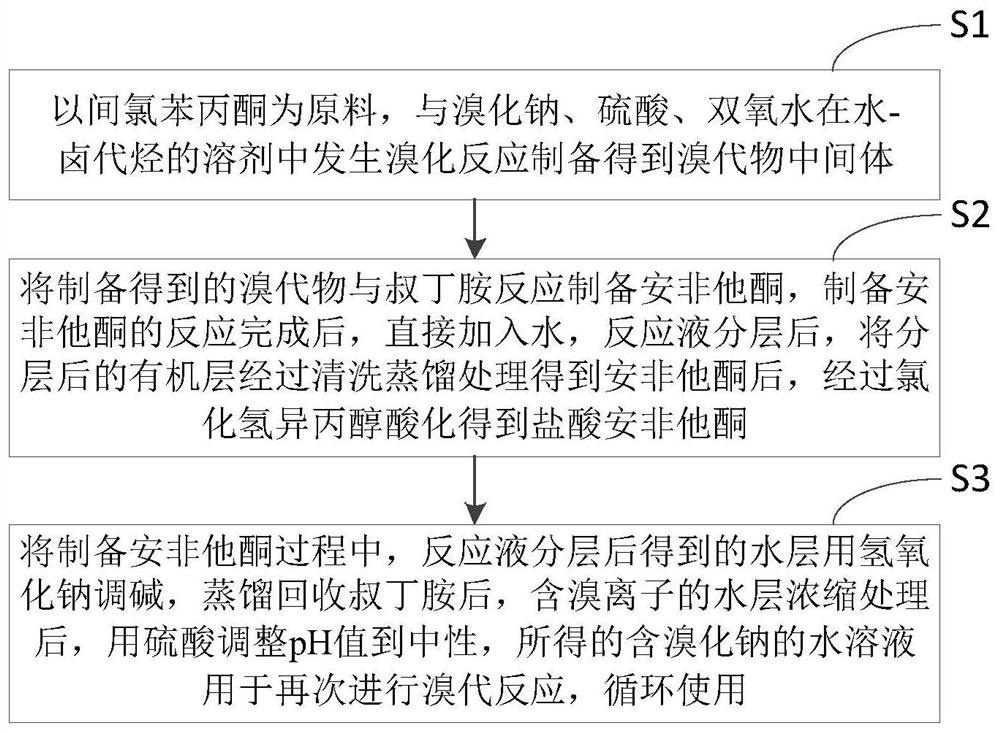
[0022] Such as figure 1 Shown, a kind of preparation method of bupropion hydrochloride of the present invention comprises the steps:
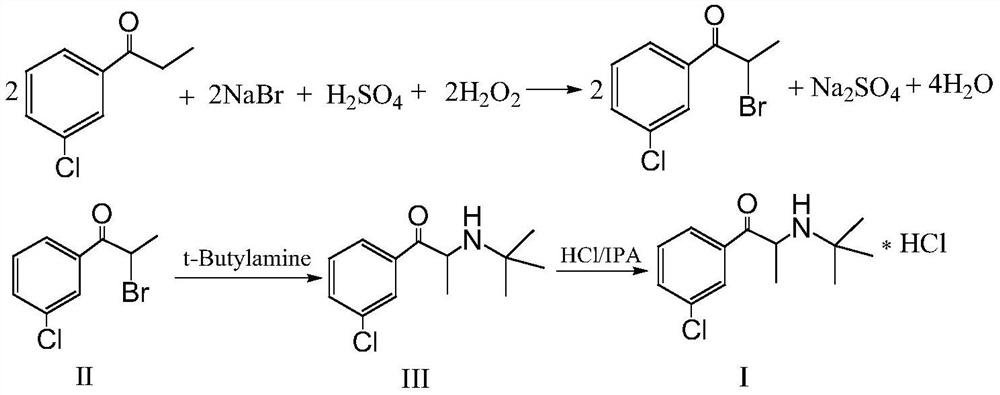
[0023] Step S1, using m-chloropropiophenone as a raw material, bromination reaction with sodium bromide, sulfuric acid, and hydrogen peroxide in a water-halogenated hydrocarbon solvent to prepare a bromide intermediate;
[0024] Step S2, reacting the prepared bromide with tert-butylamine to prepare bupropion, after the reaction for preparing bupropion is completed, directly add water, after the reaction solution is layered, the layered organic layer is cleaned and distilled to obtain After bupropion, obtain bupropion hydrochloride through hydrogen chloride isopropanol acidification;
[0025] Step S3, in the process of preparing bupropion, the water layer obtained after the reaction solution is layered is adjusted to alkali with sodium hydroxide, after distillation and recovery of tert-butylamine, the water layer containing bromide ions is conc...
Embodiment 1
[0031] At room temperature, add 30 mL of water and 15 g (0.15 mol) of concentrated sulfuric acid into the reaction flask, and lower the temperature to room temperature, add 20.6 g (0.2 mol) of sodium bromide, and stir for 20 minutes. Add 100mL of dichloromethane and 30g (0.18mol) of m-chloropropiophenone, stir until the solid is completely dissolved, raise the temperature to 40°C, slowly add 28g of 30% hydrogen peroxide (0.25mol) dropwise, and stir at 40°C for reaction 2 Hour. After the reaction, cool to normal temperature, let stand to separate layers, discard the water layer, wash the organic layer twice with 100mL water, collect the lower organic layer to obtain a dichloromethane solution of bromo-m-chloropropiophenone, and directly enter the next reaction.
[0032] At room temperature, 29 g (0.4 mol) of tert-butylamine was added to the organic layer, and the reaction solution was heated to reflux temperature for 20 hours of reaction. Cool down to room temperature after th...
Embodiment 2
[0035] At room temperature, add 30 mL of water and 20 g (0.2 mol) of concentrated sulfuric acid into the reaction flask, lower the temperature to room temperature, add 18.5 g (0.18 mol) of sodium bromide, and stir for 20 minutes. Add 130mL of dichloromethane and 30g (0.18mol) of m-chloropropiophenone, stir until the solids are completely dissolved, raise the temperature to 40°C, slowly add 34g of 30% hydrogen peroxide (0.3mol) dropwise, and stir at 40°C for reaction 2 Hour. After the reaction, cool to normal temperature, let stand to separate layers, discard the water layer, wash the organic layer twice with 100mL water, collect the lower organic layer to obtain a dichloromethane solution of bromo-m-chloropropiophenone, and directly enter the next reaction.
[0036] At room temperature, 36.5 g (0.5 mol) of tert-butylamine was added to the organic layer, and the reaction solution was heated to reflux temperature for 20 hours of reaction. Cool down to room temperature after the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com