Preparation method of oral oxiracetam preparation suitable for old people to take
A technology of binder and binder solution, which is applied in the field of preparation of oxiracetam orally disintegrating tablets, can solve the problems of uneven particles, narrow particle size distribution, poor compressibility, etc. Small and uniform effect with good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Oxiracetam Orally Disintegrating Tablets
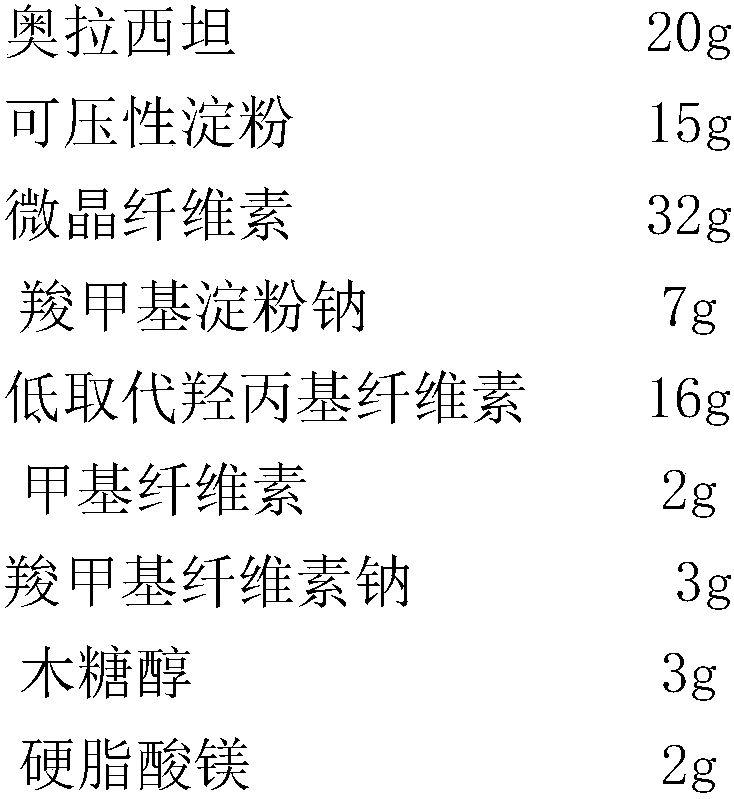
[0025] Prescription (mass ratio)
[0026]
[0027] Preparation:
[0028](1) Material screening: Oxiracetam, compressible starch, microcrystalline cellulose, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose, sodium carboxymethyl cellulose, methyl cellulose, wood Sugar alcohol and magnesium stearate are passed through a 100-mesh sieve respectively, and set aside;
[0029] (2) Fluidized bed granulation: add oxiracetam, filler, and disintegrant into the fluidized bed granulator, and mix with the air, where the air temperature is 45°C, and the air velocity is 1100m 3 / hour; raise the inlet air temperature, spray the adhesive solution in the top spray mode, mix and dry under the condition of continuous air inlet, and obtain oxiracetam granules, wherein the inlet air velocity is 1300m 3 / hour, the air inlet temperature is 75°C, the concentration of the binder solution is 8% in g / mL, and the i...
Embodiment 2
[0032] Preparation of Oxiracetam Orally Disintegrating Tablets
[0033] Prescription (mass ratio)
[0034]
[0035]
[0036] Preparation:
[0037] (1) Material screening: Oxiracetam, compressible starch, microcrystalline cellulose, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose, methyl cellulose, sodium carboxymethyl cellulose, Acesulfame K and magnesium stearate are respectively passed through a 100-mesh sieve, and set aside;
[0038] (2) Fluidized bed granulation: Add oxiracetam, filler, and disintegrating agent into the fluidized bed granulator, and mix with the air, where the air temperature is 50°C, and the air velocity is 1200 3 / hour; raise the inlet air temperature, spray the binder solution in the top spray mode, mix and dry under the condition of continuous air inlet, and obtain oxiracetam granules, wherein the inlet air velocity is 1400m 3 / hour, the air inlet temperature is 90°C, the concentration of the binder solution is 10% in g / ...
Embodiment 3
[0041] Preparation of Oxiracetam Orally Disintegrating Tablets
[0042] Prescription (mass ratio)
[0043]
[0044] Preparation:
[0045] (1) Material screening: Oxiracetam, compressible starch, microcrystalline cellulose, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose, methyl cellulose, sodium carboxymethyl cellulose, ammonium Sieve honey and micropowder silica gel through a 100-mesh sieve respectively, and set aside;
[0046] (2) Fluidized bed granulation: add oxiracetam, filler, and disintegrant into the fluidized bed granulator, and mix with the air, where the air temperature is 38°C, and the air velocity is 1000 3 / hour; increase the temperature of the air inlet, spray the adhesive solution in the way of side spray, mix and dry under the condition of continuous air inlet, and obtain oxiracetam particles, wherein the air inlet speed is 1200m 3 / hour, the air inlet temperature is 68°C, the concentration of the binder solution is 6% in g / mL, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com