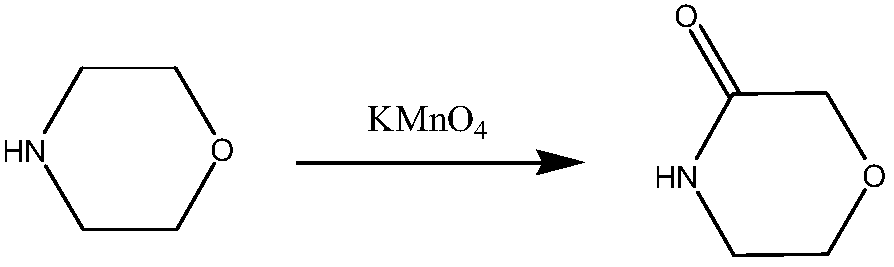
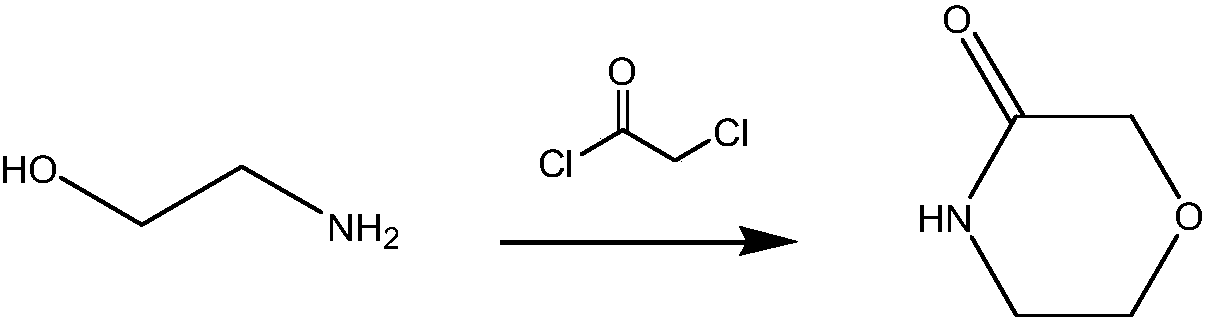
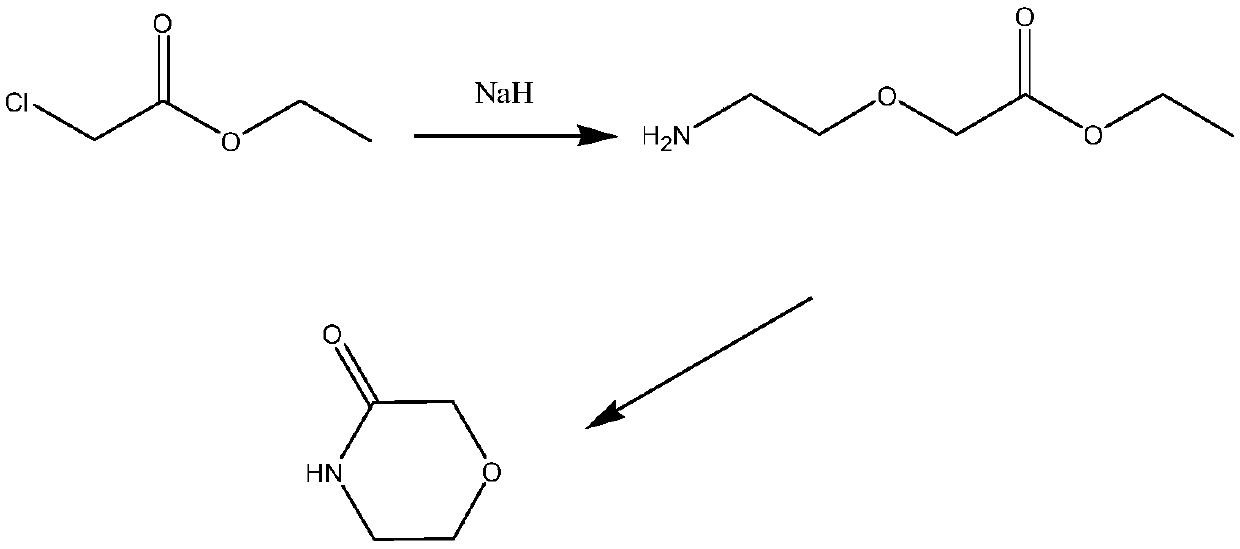
A kind of preparation method of 3-morpholinone
A technology of morpholinone and hydroxyacetate, which is applied in the field of preparation of 3-morpholinone, can solve problems such as process safety hazards, difficulty in batch production, and severe reactions, and achieve high purity, less side reactions, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The invention provides a kind of preparation method of 3-morpholinone, comprises the following steps:
[0026] (1) Dehydrohalogenation of 2-haloethylamine hydrohalide in the presence of an organic solvent, after mixing the resulting reaction system with 2-hydroxyacetate, carry out amine transesterification to obtain 2-(2 - glycolamido) haloethane;
[0027] (2) The 2-(2-hydroxyacetamido) haloethane in the step (1) is subjected to a ring-closing reaction in the presence of an alkali reagent and an organic solvent to obtain 3-morpholinone.
[0028] In the present invention, 2-haloethylamine hydrohalide is dehydrohalogenated in the presence of an organic solvent, and the resulting reaction system is mixed with 2-hydroxyacetate to perform amine transesterification to obtain 2-(2- Glycolamido) haloethanes.
[0029] In the present invention, the 2-haloethylamine hydrohalide preferably includes 2-chloroethylamine hydrochloride or 2-bromoethylamine hydrobromide. In the presen...
Embodiment 1
[0047] Stir and mix 116g (1.0mol) of 2-chloroethylamine hydrochloride with 300mL of dichloromethane, cool in an ice-water bath to 10°C, add 40g of sodium hydroxide (1.0mol) in batches under nitrogen protection, and control the temperature of the system not to exceed 20°C, after adding, stir for 30 minutes to dehydrochloride; add 114.5 g (1.1 mol) of ethyl 2-hydroxyacetate dropwise to the obtained reaction system containing 2-chloroethylamine, after dropping, react at room temperature for 9 hours, GC detection 2 - Chloroethylamine is completely reacted; the resulting system is filtered, the filtrate obtained is washed with 100 mL of water, the aqueous phase in the material obtained after washing is separated, the aqueous phase obtained is extracted with 100 mL of dichloromethane, and the organic phase obtained is After drying with anhydrous sodium sulfate and distilling off the solvent under reduced pressure, 2-(2-hydroxyacetamido)chloroethane was obtained.
[0048] Mix the 2-(...
Embodiment 2
[0051] Stir and mix 205g (1.0mol) of 2-chloroethylamine bromate and 800mL of dichloromethane, cool in an ice-water bath at 15°C, add 138.2g of potassium carbonate (1.0mol) in batches under nitrogen protection, and control the temperature of the system not to exceed 20°C, after adding, stir for 30 minutes to remove hydrogen bromide; add 156 g (1.5 mol) of ethyl 2-hydroxyacetate dropwise to the obtained reaction system containing 2-bromoethylamine, after dropping, react at room temperature for 9 hours, and detect by GC The reaction of 2-bromoethylamine is complete; the resulting system is filtered, and the filtrate obtained is washed with 200 mL of water, the water phase in the material obtained after washing is separated, and the obtained water phase is extracted with 150 mL of dichloromethane, and the obtained organic The phase was dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 2-(2-hydroxyacetamido)bromoethane.
[0052]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com