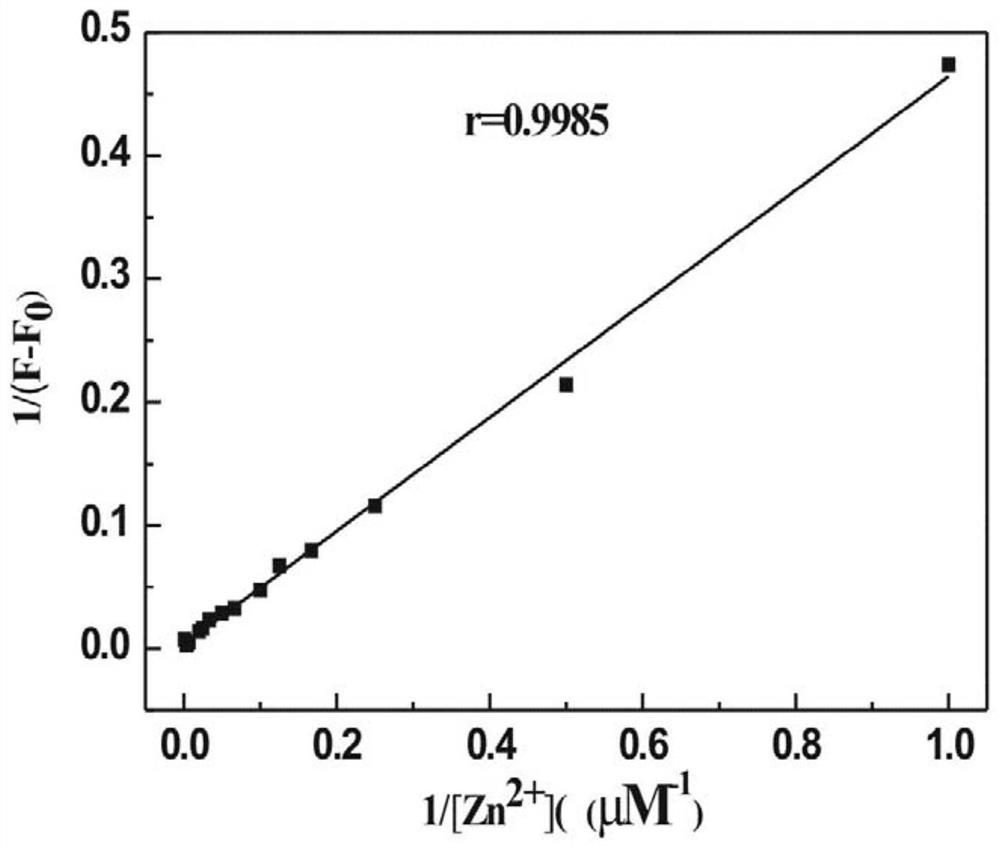
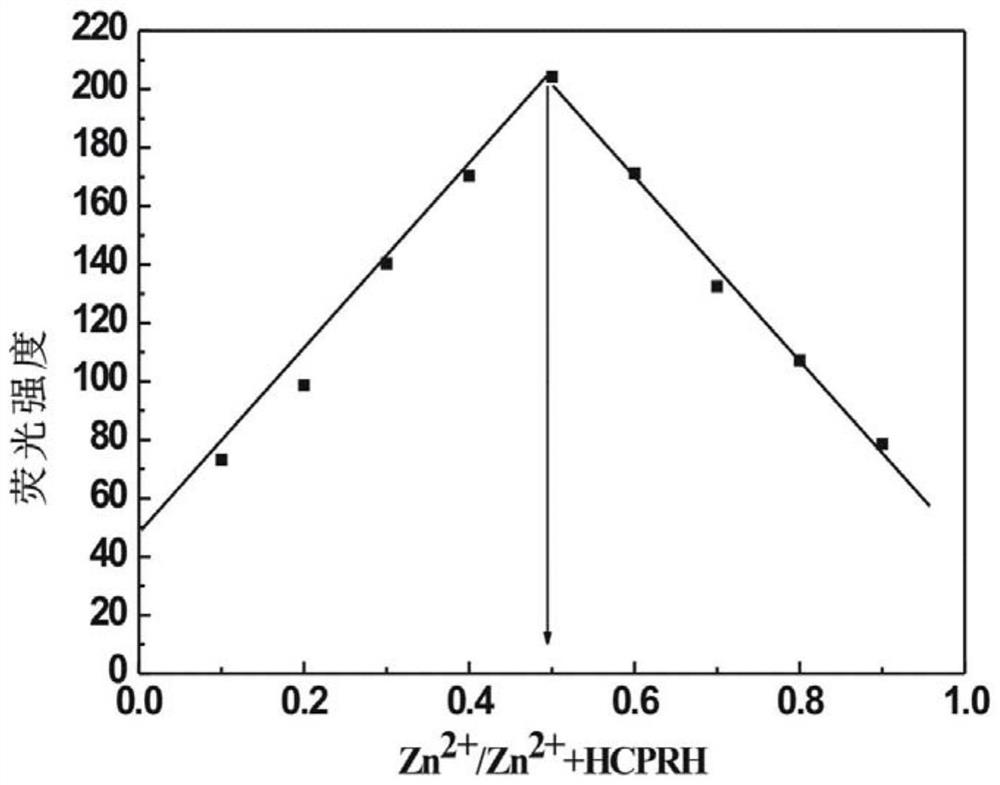
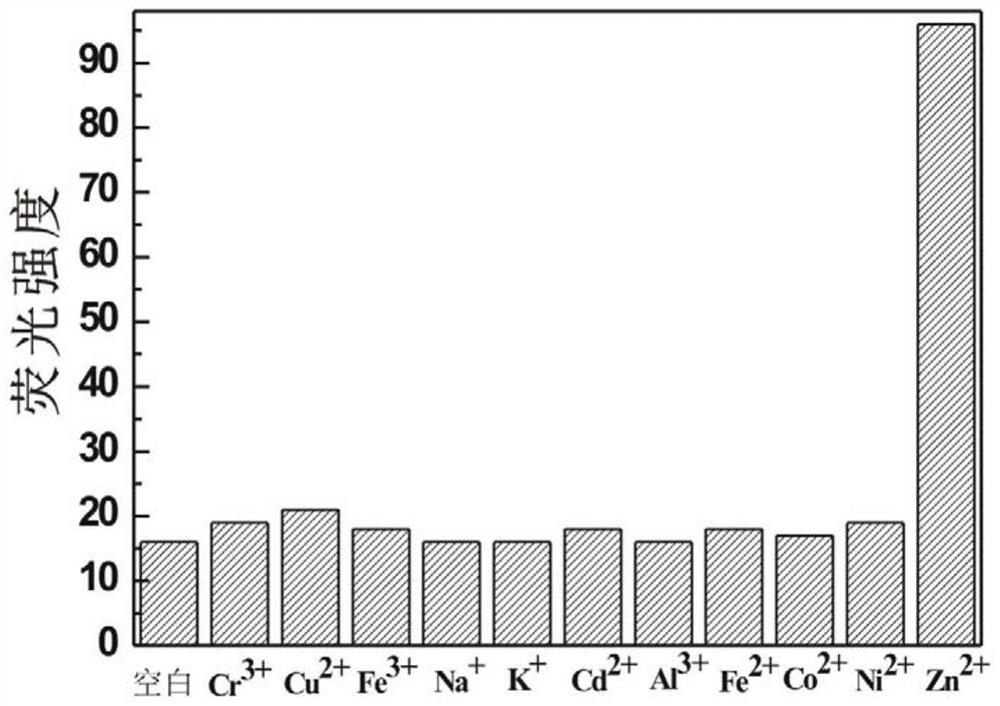
Preparation and application of a kind of n-(2-hydroxyl-5-chlorophenyl)ylrhodamine b hydrazide
The technology of hydrazide and chlorobenzene is applied in the synthesis field of simple preparation of rhodamine B derivative fluorescent sensing materials, which can solve the problems of complex identification and detection methods of zinc ions, low sensitivity of identification response, expensive instruments, etc. The effect of low dosage, good identification selectivity and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Dissolve 1.2g (2.5mmol) of Rhodamine B in ethanol solvent, add 2mL of 80% hydrazine hydrate, heat and reflux at 80°C for 3 hours, concentrate the solvent, and precipitate a yellow product, filter it with suction, dry it and put it in In a small beaker, continuously drop concentrated hydrochloric acid to dissolve the product completely into a purple-red solution, then adjust the pH value of the system to about 7.0 with 40% aqueous sodium hydroxide solution, and a large amount of light pink solid is precipitated. After suction filtration and drying, light pink rhodamine is obtained. B hydrazide intermediate product powder;
[0027] (2) Dissolve 0.456g (1mmol) of the intermediate product in step (1) in methanol and add 0.133g (0.8mmol) of 5-chlorosalicylaldehyde. After refluxing at 50°C for 28 hours, a white solid precipitates and cools to room temperature. Afterwards, suction filtration, washing, vacuum drying at 40°C for 12 hours, and finally recrystallization twice ...
Embodiment 2
[0029] (1) Dissolve 1.2g (2.5mmol) of rhodamine B in ethanol solvent, add 4mL of 80% hydrazine hydrate, heat and reflux at 90°C for 1 hour, concentrate the solvent, and precipitate a yellow product, filter it with suction, dry it and put it in In a small beaker, continuously drop concentrated hydrochloric acid to dissolve the product completely into a purple-red solution, then adjust the pH value of the system to about 7.0 with 40% aqueous sodium hydroxide solution, and a large amount of light pink solid is precipitated. After suction filtration and drying, light pink rhodamine is obtained. B hydrazide intermediate product powder;
[0030] (2) Dissolve 0.456g (1mmol) of the intermediate product in step (1) in methanol and add 0.199g (1.2mmol) of 5-chlorosalicylaldehyde. After refluxing at 70°C for 20 hours, a white solid precipitates and cools to room temperature. Afterwards, suction filtration, washing, vacuum drying at 50°C for 8 hours, and finally recrystallization twice wi...
Embodiment 3
[0032] (1) Dissolve 1.2g (2.5mmol) of rhodamine B in ethanol solvent, add 3mL of 80% hydrazine hydrate, heat and reflux at 85°C for 2 hours, concentrate the solvent, and precipitate a yellow product, filter it with suction, dry it and put it in In a small beaker, continuously drop concentrated hydrochloric acid to dissolve the product completely into a purple-red solution, then adjust the pH value of the system to about 7.0 with 40% aqueous sodium hydroxide solution, and a large amount of light pink solid is precipitated. After suction filtration and drying, light pink rhodamine is obtained. B hydrazide intermediate product powder;
[0033] (2) Dissolve 0.456g (1mmol) of the intermediate product in step (1) in methanol and add 0.166g (1.0mmol) of 5-chlorosalicylaldehyde. After reflux at 60°C for 24 hours, a white solid is precipitated and cooled to room temperature. Afterwards, suction filtration, washing, vacuum drying at 45°C for 10 hours, and finally recrystallization twice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com