Triarylethene photochromic material containing furan derivatives and its synthesis method and application
A photochromic material, triarylethylene technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of high material cost, easy oxidation cycle times, complicated design and synthesis, etc. Excellent rewritability, excellent development potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The above-mentioned synthetic method of the triarylethylene-based photochromic material containing furan derivatives, comprising the following methods:
[0030] Method (1): Synthesize the dibromo-substituted product of the furan ring or its derivative containing a formaldehyde substituent at one end through the Corey-Funchs reaction; The target product is obtained by Suzuki reaction. Preferably, the method (1) includes the following steps: 1. providing a furan ring containing a formaldehyde substituent at one end or a derivative thereof, in a dichloromethane solution, under the action of triphenylphosphine and carbon tetrabromide, at room temperature The reaction obtains its dibromo substituted product; ② the dibromo substituted product obtained in step 1 is combined with an aromatic compound or heterocyclic compound containing boronic acid or pinacol boronate group in a tetrahydrofuran solution, under the action of potassium carbonate, through tetratriphenylene The ta...
Embodiment 1
[0035] (1) Synthesis of intermediate [2-(2,2-dibromoethylene)furan]
[0036]
[0037] In a 250ml single-necked flask, add 60ml of dichloromethane, 4.76g of carbon tetrabromide (20mmol) and 10.49g of triphenylphosphine (40mmol), place in an ice-water bath, and add 0.96g of 2-furan after reacting on a magnetic stirrer for 1h Formaldehyde (10mmol) was reacted at room temperature for 16h. A black solution was obtained, and the excess solvent was evaporated in a rotary evaporator to obtain an oily mixed product. Then, it is purified by silica gel column chromatography, and the eluent is n-hexane. 2.28 g of white solid were obtained, and the yield was 91.31%.
[0038] (2) Synthesis of target product Example 1
[0039]
[0040] Add 1.25g 2-(2,2-dibromoethylene) furan (5mmol) and 1.68g phenylboronic acid (15mmol) in a 250mL there-necked flask, add an appropriate amount of THF in a nitrogen atmosphere, add 3.45g K 2 CO 3 (15.00mmol) in 2mol / L aqueous solution, add 5‰ catalys...
Embodiment 2
[0042]
[0043] Referring to the step (2) of Example 1, 4-fluorophenylboronic acid was used to synthesize the target product Example 2, and the yield was 88.24%. 1 H NMR (500MHz, DMSO-d 6 )δ7.52(d,J=1.4Hz,1H),7.35-7.27(m,4H),7.26-7.19(m,2H),7.19-7.12(m,2H),7.01(s,1H),6.37 –6.31(m,1H),5.55(d,J=3.4Hz,1H).
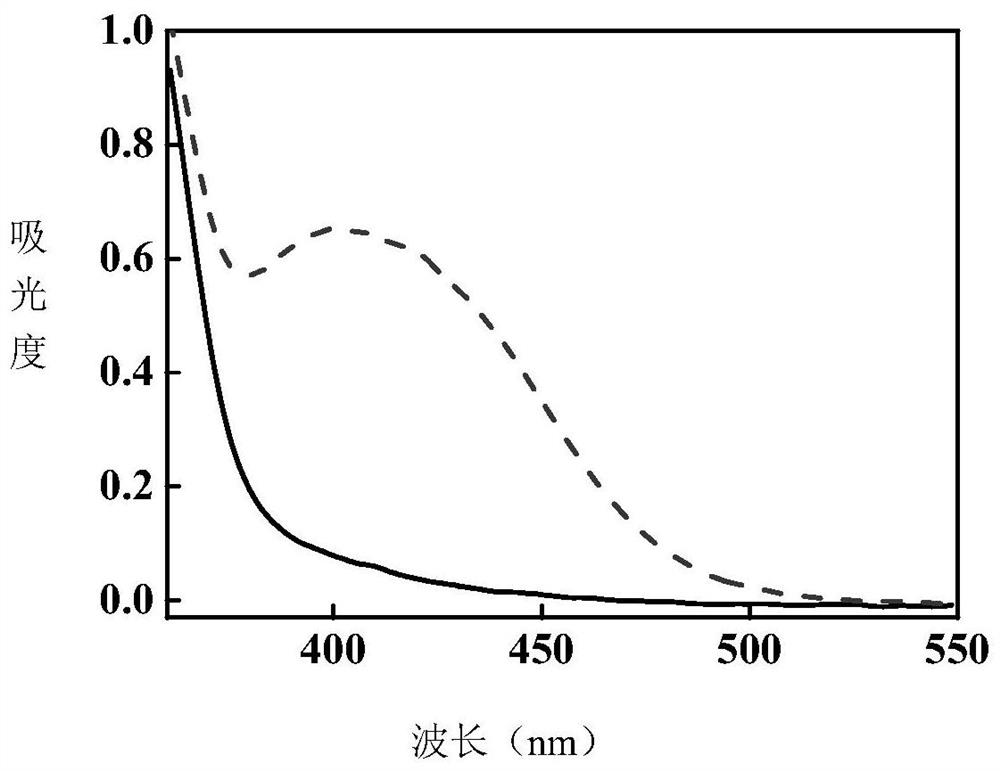
[0044] The product of this example is formulated to a concentration of 1.0×10 -3 M tetrahydrofuran solution, under the irradiation of 365nm LED ultraviolet light source, gradually turned yellow from a colorless and transparent solution, and measured the comparison of ultraviolet absorption spectra before and after photochromic figure 1 shown.
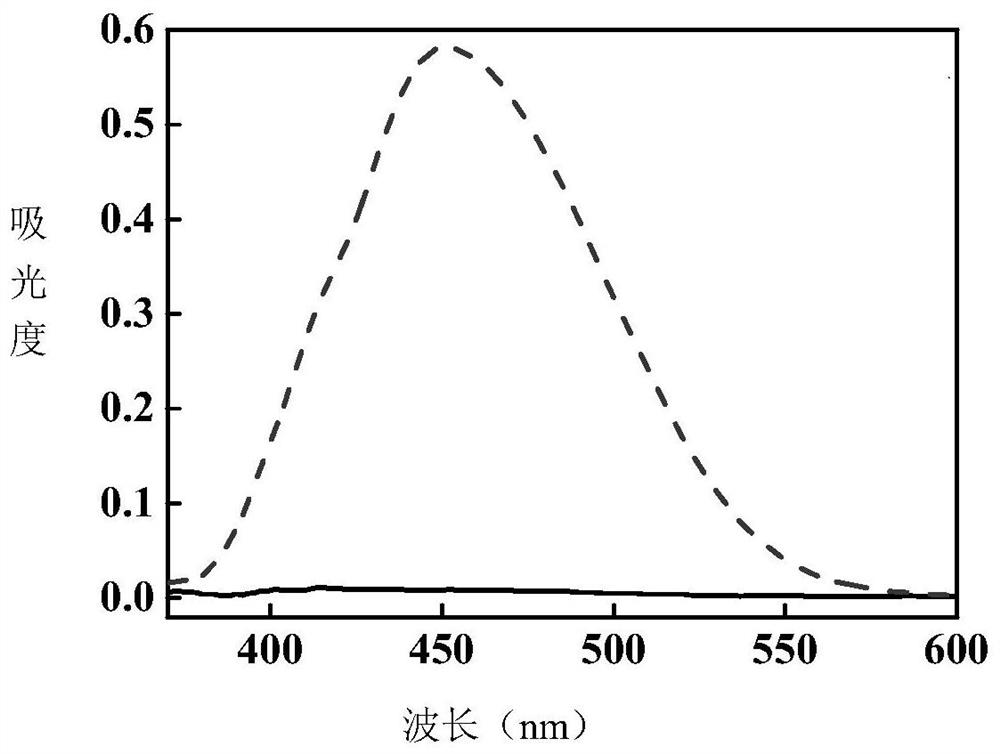
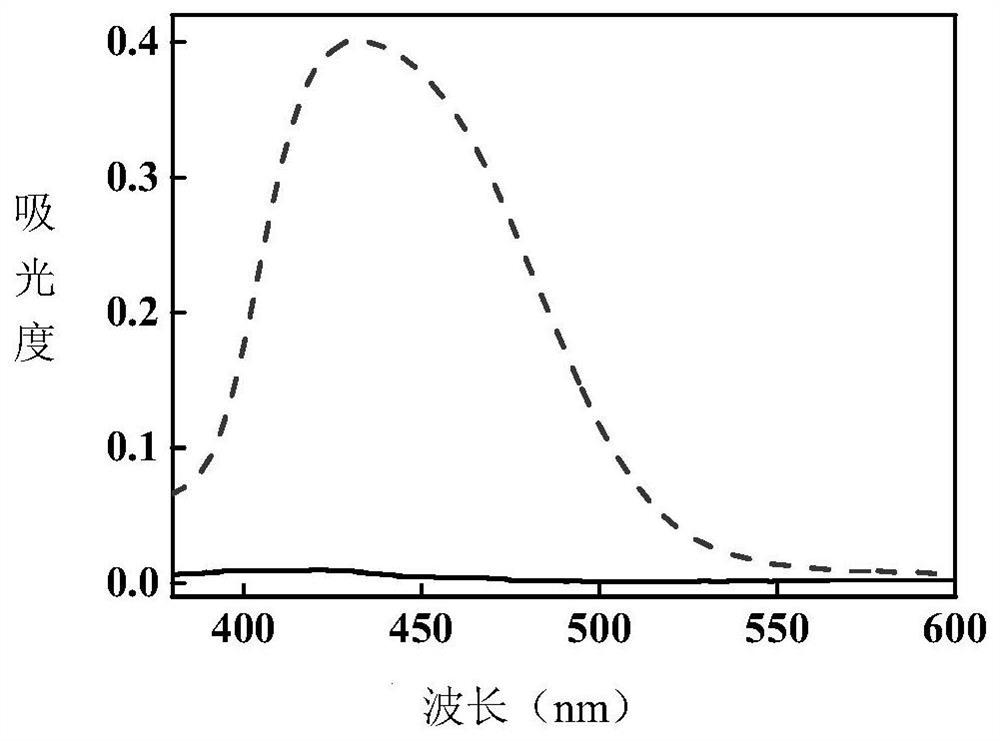
[0045] Under the irradiation of 365nm LED ultraviolet light source, the product of this example gradually turned yellow from white crystal powder. The comparison of ultraviolet absorption spectra before and after photochromic was measured. figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com