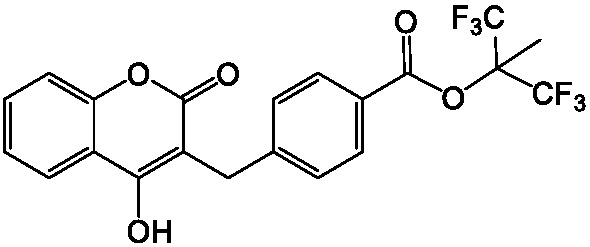
Tecarfarin preparation method
A compound and catalyst technology, applied in the field of preparation of Tecarfarin, can solve the problems of unsuitability for large-scale industrial production, expensive condensing agent, severe reaction conditions, etc., and achieve good industrial application prospects, high yield and high controllability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
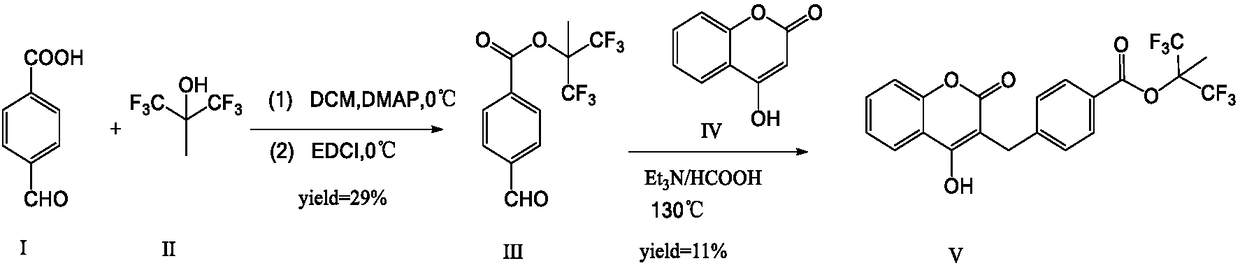
Examples
Embodiment 1
[0030]
[0031] Under N2 protection, 1 (2.0 g, 1.0 eq) was suspended in anhydrous DCM (20 ml), and DMF (0.05 ml) was added. Oxalyl chloride (3.3g, 2.0eq) was added dropwise at 0-10°C, dissolved, stirred for 10min, kept at 0-10°C for 8h. Sample TLC. After the reaction was completed, it was concentrated to dryness under reduced pressure, and the concentrate was dissolved in anhydrous DCM for later use.
[0032] 3 (2.4g, 1.0eq) was dissolved in anhydrous DCM, triethylamine (1.75g, 1.3eq) was added, stirred at 0-10°C for 10min. Slowly add the above acid chloride solution dropwise. After dropping, stir at 25-30°C for 4h. After the reaction was completed, it was concentrated to dryness under reduced pressure, and water was added to dissolve EA. The layers were separated, the aqueous layer was extracted with EA, and the organic layers were combined. Wash with 10% citric acid, twice with dilute sodium hydroxide solution, and saturated brine, and concentrate under reduced press...
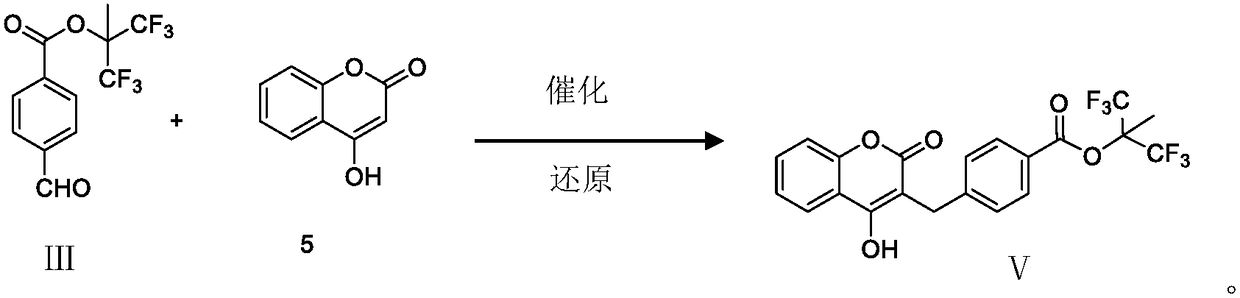
Embodiment 2
[0034]
[0035] Dissolve III, 5 in THF and stir for 10 min. Add 6,7,20-30 ℃ and keep stirring for 12h. After the reaction was completed, it was concentrated under reduced pressure, EA was dissolved, and washed with 1N NaOH solution. Separate the liquid, remove the organic layer, adjust the pH to 5-6 with 1N HCl, and extract with EA. Separate the liquid and remove the aqueous layer. Washed with saturated sodium bicarbonate solution, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain a white solid, which was recrystallized from acetone to obtain the target product V. Yield: 60%
Embodiment 3
[0037]
[0038] Under N2 protection, 1 (1.0eq) was suspended in anhydrous THF, and DMF was added. Add oxalyl chloride (2.0eq) dropwise at 0-10°C, dissolve, stir for 10min, keep stirring at 0-10°C for 8h. Sample TLC. After the reaction was completed, it was concentrated to dryness under reduced pressure, and the concentrate was dissolved in anhydrous tetrahydrofuran for later use.
[0039] Dissolve 3 (1.0eq) in anhydrous tetrahydrofuran, add pyridine (1.3eq), and stir at 0-10°C for 10min. Slowly add the above acid chloride solution dropwise. After dropping, stir at 25-30°C for 4h. After the reaction was completed, it was concentrated to dryness under reduced pressure, and water was added to dissolve EA. The layers were separated, the aqueous layer was extracted with EA, and the organic layers were combined. Wash with 10% citric acid, twice with sodium hydroxide solution, and with saturated brine, and concentrate under reduced pressure to dryness to obtain pale yellow so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com