Novel triamcinolone acetonide high-polymer medicinal long-acting slow-release membrane and preparation method thereof
A polymer and slow-release film technology, applied in the fields of polymer materials, biomedicine, and organic chemistry, can solve the problems of drug washing and dilution, difficulties in local administration and repeated administration, and impact on bile duct healing, etc., to achieve Avoid systemic hormone side effects, reduce the risk of reoperation, good physical and chemical properties and biological properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
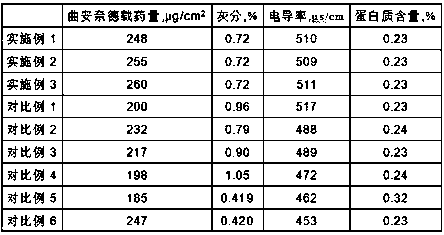
Examples
Embodiment 1
[0047] A preparation method of a novel triamcinolone acetonide macromolecule drug long-acting slow-release membrane, comprising the steps of:
[0048] Step (1), purification of chitosan raw material:
[0049] Chitosan was added to an aqueous sodium hydroxide solution with a concentration of 40 g / L, stirred at 70° C. to decolorize and deproteinize, and then separated from solid and liquid, and the solid was taken to obtain purified chitosan;
[0050] Wherein, the add-on ratio of chitosan and sodium hydroxide aqueous solution is 20g: 100ml;
[0051] Step (2), screening chitosan with high degree of deacetylation:
[0052] Place the purified chitosan obtained in step (1) in a mass concentration of 1% dilute hydrochloric acid and stir until dissolved, filter, take the filtrate, slowly add 1mol / L sodium hydroxide solution to the filtrate until the pH value is 7.6, Filtrate, get filter residue, wash to neutrality, obtain chitosan with high degree of acetylation;
[0053] The quali...
Embodiment 2
[0068] A preparation method of a novel triamcinolone acetonide macromolecule drug long-acting slow-release membrane, comprising the steps of:
[0069] Step (1), purification of chitosan raw material:
[0070] Chitosan was added to an aqueous sodium hydroxide solution with a concentration of 40 g / L, stirred at 70°C for 2 hours to decolorize and deproteinize, and then separated from solid and liquid, and the solid was taken to obtain purified chitosan;
[0071] Wherein, the add-on ratio of chitosan and sodium hydroxide aqueous solution is 20g: 100ml;
[0072] Step (2), screening chitosan with high degree of deacetylation:
[0073] Put the purified chitosan obtained in step (1) in dilute hydrochloric acid with a mass concentration of 1% and stir for 2 hours, filter, take the filtrate, slowly add 1mol / L sodium hydroxide solution to the filtrate until the pH value is 7.9, filter , take filter residue, wash to neutrality, obtain chitosan with high degree of acetylation;
[0074] ...
Embodiment 3
[0090] A preparation method of a novel triamcinolone acetonide macromolecule drug long-acting slow-release membrane, comprising the steps of:
[0091] Step (1), purification of chitosan raw material:
[0092] Chitosan was added to an aqueous sodium hydroxide solution with a concentration of 40 g / L, stirred at 70°C for 2 hours to decolorize and deproteinize, and then separated from solid and liquid, and the solid was taken to obtain purified chitosan;
[0093] Wherein, the add-on ratio of chitosan and sodium hydroxide aqueous solution is 20g: 100ml;
[0094] Step (2), screening chitosan with high degree of deacetylation:
[0095] Put the purified chitosan obtained in step (1) in dilute hydrochloric acid with a mass concentration of 1% and stir for 2 hours, filter, take the filtrate, slowly add 1mol / L sodium hydroxide solution to the filtrate until the pH value is 7.8, filter , take filter residue, wash to neutrality, obtain chitosan with high degree of acetylation;
[0096] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com