Vanadium-free denitration catalyst and preparation method thereof
A denitration catalyst, a technology of chemical composition, applied in chemical instruments and methods, chemical elements of heterogeneous catalysts, catalysts for physical/chemical processes, etc., to achieve the effects of simple production process, high catalytic performance and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
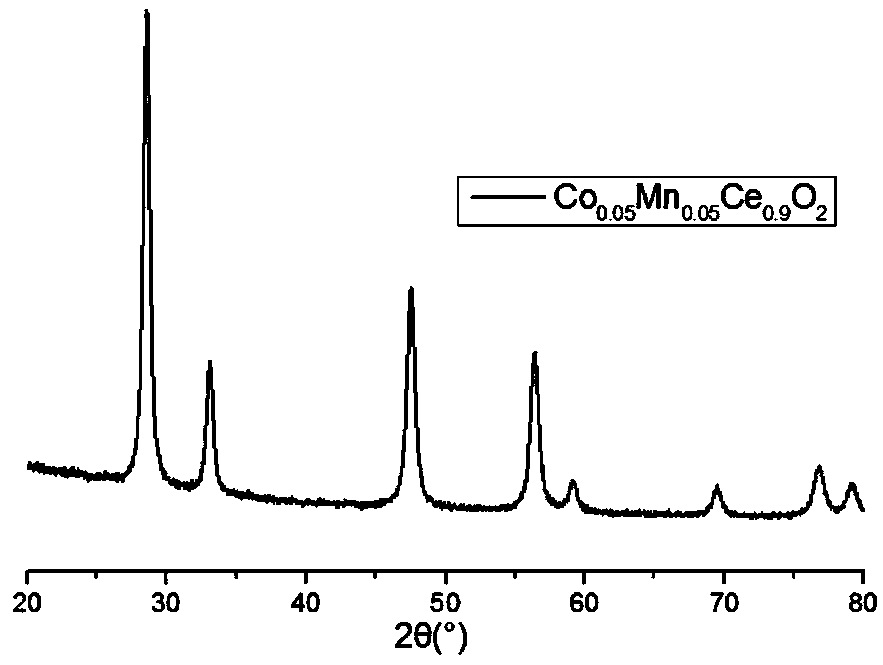
[0035] Dissolve cerium nitrate (0.3907g), manganese nitrate (12.55mg) and cobalt nitrate (14.55mg) in (20mL) water, where Ce 3+ with Co 2+ , Mn 2+ The molar ratio of the mixture was 18:1:1, stirred at 800rpm at room temperature for 30min to make it evenly mixed. Dissolve biphenyldicarboxylic acid (0.242g) in (10mL) ethanol, mix the two solutions, heat in an oil bath at 70°C, use sodium hydroxide as a regulator, adjust the pH of the solution to 4, and keep the temperature constant for 2h. The chelation reaction product was centrifuged and dried, and dried in an oven at 60°C. The dried sample was placed in a muffle furnace and sintered at 600°C for 2 hours. The obtained vanadium-free denitration catalyst was a Co and Mn-doped CeO 2 Composite oxide catalyst, its SEM such as figure 1 As shown, the XRD pattern is as figure 2 As shown, the yield was 83%.
[0036] Depend on Figure 1-2 It can be seen that the impurity elements in the obtained doped oxide are uniformly doped in...
Embodiment 2
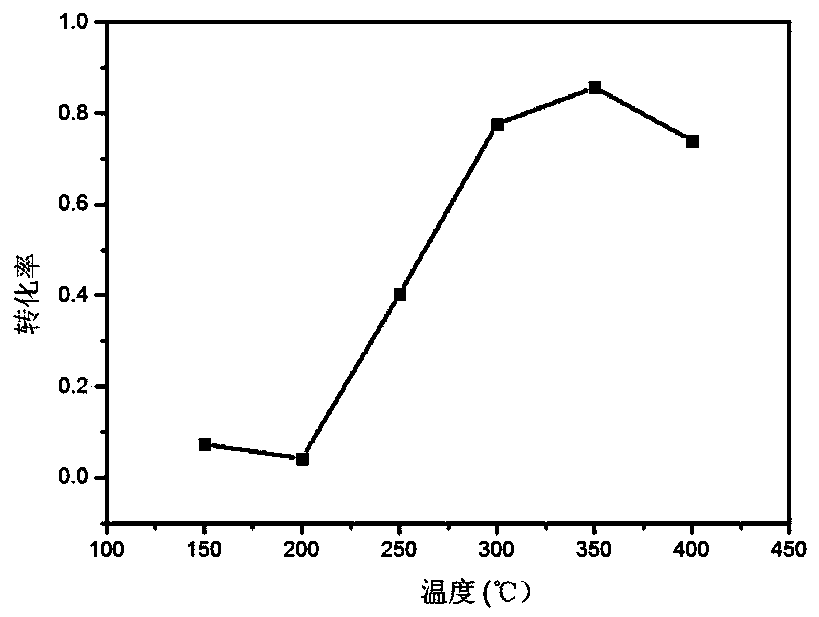
[0040] Dissolve cerium nitrate (0.3038g) and manganese nitrate (75mg) in 10ml of water, where Ce 3+ with Mn 2+ The molar ratio of the mixture was 7:3, stirred at 500rpm at room temperature for 30min to make it evenly mixed. Dissolve 0.166g of phthalic acid in (30ml) ethanol, mix the two solutions, heat in an oil bath at 50°C, use sodium hydroxide as a pH regulator, adjust the pH of the solution to 3, and keep the temperature constant for 2h. The chelation reaction product was centrifuged and dried in an oven at 60°C, and the dried sample was placed in a muffle furnace and sintered at 400°C for 4h to obtain Mn / CeO 2 Composite vanadium-free denitration catalyst with a yield of 70%. The denitration efficiency of the denitration catalyst prepared in the embodiment of the present invention is 90%.
Embodiment 3
[0042] Dissolve cerium nitrate (0.3472g) and cobalt nitrate (58.2mg) in 10ml of water, where Ce 3+ with Co 2+ The molar ratio of the mixture was 8:2, stirred at 800rpm at room temperature for 30min to make it evenly mixed. Dissolve 0.166g of phthalic acid in 40ml of ethanol, mix the two solutions, heat in an oil bath at 50°C, use sodium hydroxide as a regulator, adjust the pH of the solution to 4, and keep the temperature constant for 2h. The reactant was centrifuged and dried in an oven at 60°C, and the dried sample was placed in a muffle furnace and sintered at 500°C for 3 hours to obtain Co / CeO 2 Composite vanadium-free denitration catalyst with a yield of 80%. The denitration efficiency of the denitration catalyst prepared in the embodiment of the present invention is 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com