A fluorescent probe, synthesis method and application for detecting peroxynitrite anion
A technology of peroxynitroso group and synthesis method is applied in the field of synthesizing and detecting fluorescent probes of peroxynitrite anion, which can solve the problems of interference, low selectivity and small Stoke shift, and achieve accurate detection and selection. High-performance, easy-to-synthesize effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] About the synthetic method of fluorescent probe, comprise following experimental steps:
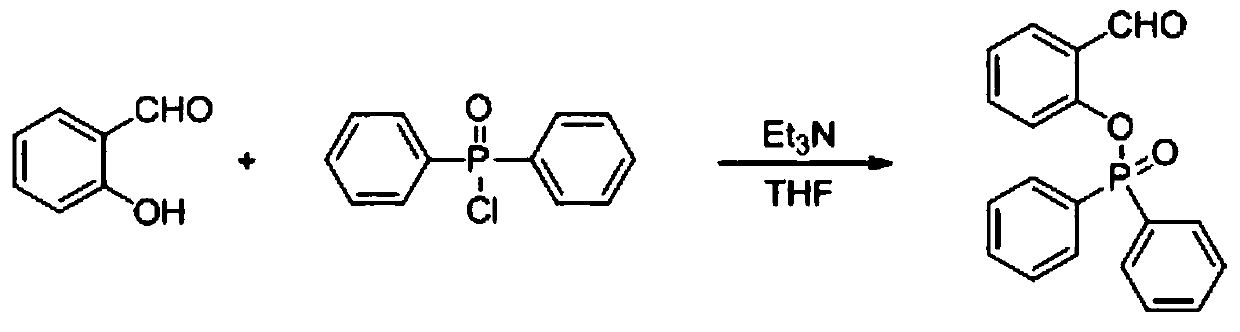
[0034] (1) 2-Hydroxybenzaldehyde (122mg, 1mmol) and triethylamine (200mg, 2mmol) were dissolved in dry 10mL tetrahydrofuran, and diphenylphosphinic chloride (236.5mg, 1mmol) was added dropwise under stirring, at room temperature Down reaction 12 hours;
[0035] (2) After the reaction is completed, evaporate under reduced pressure to obtain the crude product, and then use silica gel column chromatography, wherein the volume ratio of ethyl acetate and sherwood oil in the silica gel column chromatography is 1:4 to obtain a white intermediate product (232mg, Productive rate 72%), the synthetic reaction equation of this intermediate product is as figure 1 shown;
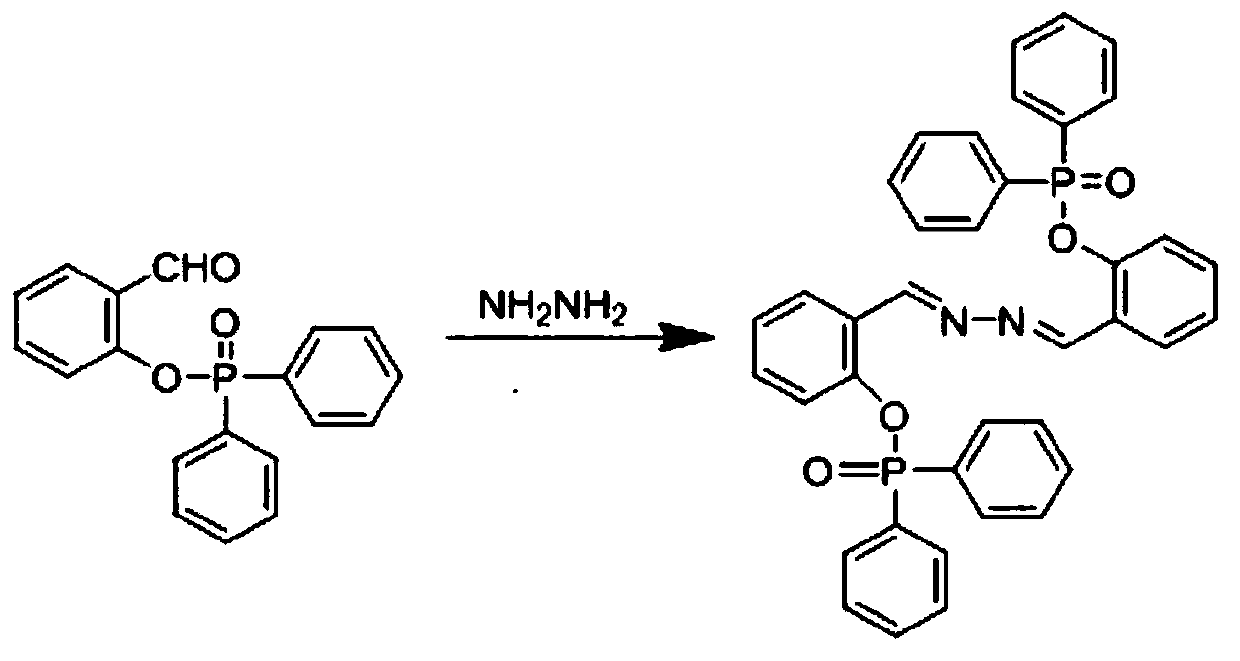
[0036] (3) Dissolve the white intermediate product (322mg, 1mmol) and hydrazine hydrate (50mg, 1mmol) in 10mL ethanol, the reaction mixture is heated, stirred, and refluxed for 2 hours. After the reaction solution is cooled...
Embodiment 2
[0041] About testing fluorescent probe detection ONOO - effect, the experimental steps are:
[0042](1) Dissolving the fluorescent probe in N,N-dimethylformamide (DMF) to prepare a 1mmol / L probe solution;
[0043] (2) Take the probe solution and add DMF and PBS (pH = 7.4) buffer solution to form a 10 μM solution (organic phase: PBS aqueous phase = 1:99, V / V), and test the changes in the ultraviolet absorption spectrum and fluorescence emission spectrum Condition.
[0044] The changes in the UV absorption spectrum are as follows: Figure 5 As shown, where the fluorescent probe was added to ONOO - The UV-Vis absorption spectrum curve is (a), without ONOO - The UV-visible absorption spectrum curve is (b). From Figure 5 It can be seen from the figure that when ONOO is not added - In the case of , the probe has no absorption peak at 400nm, while adding ONOO - Afterwards, the probe has an obvious absorption peak at 400nm, indicating that this fluorescent probe has a strong ...
Embodiment 3
[0047] In order to test the Stoke shift value of the fluorescent probe of the present invention, the following experiment is now done.
[0048] (1) Synthesis of ONOO by autoxidation of hydroxylamine in alkaline medium - : Vigorously stir the mixed solution containing 0.01mol / L hydroxylamine, 0.5mol / LNaOH and 0.001mol / L EDTA under aerobic conditions for about 3 hours, then use MnO 2 Powder filtration mixture to remove H 2 o 2 , The filtered mixture can be used for experiments immediately or stored at -18°C. Among them, ONOO can be detected at 302nm by UV-Vis spectrophotometer - concentration.
[0049] (2) Dissolve the fluorescent probe in N,N-dimethylformamide (DMF) to prepare a 1mmol / L probe solution, add the probe solution to DMF and PBS (pH=7.4) buffer, and prepare into a 10uM (organic phase: PBS aqueous phase = 1:99, V / V) solution; then add 45uM ONOO - The reaction solution was delayed for 10 seconds, and its luminous intensity was continuously measured for 10 seconds...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com