A method for constructing a cell model for screening antioxidant drugs based on protein misfolding
A cell model and misfolding technology, applied in the field of cell biology, can solve problems such as misfolding and inability to quantitatively evaluate, and achieve high chromosome integration, sensitivity to antioxidant drugs, and stable genetic traits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Packaging lentiviral particles
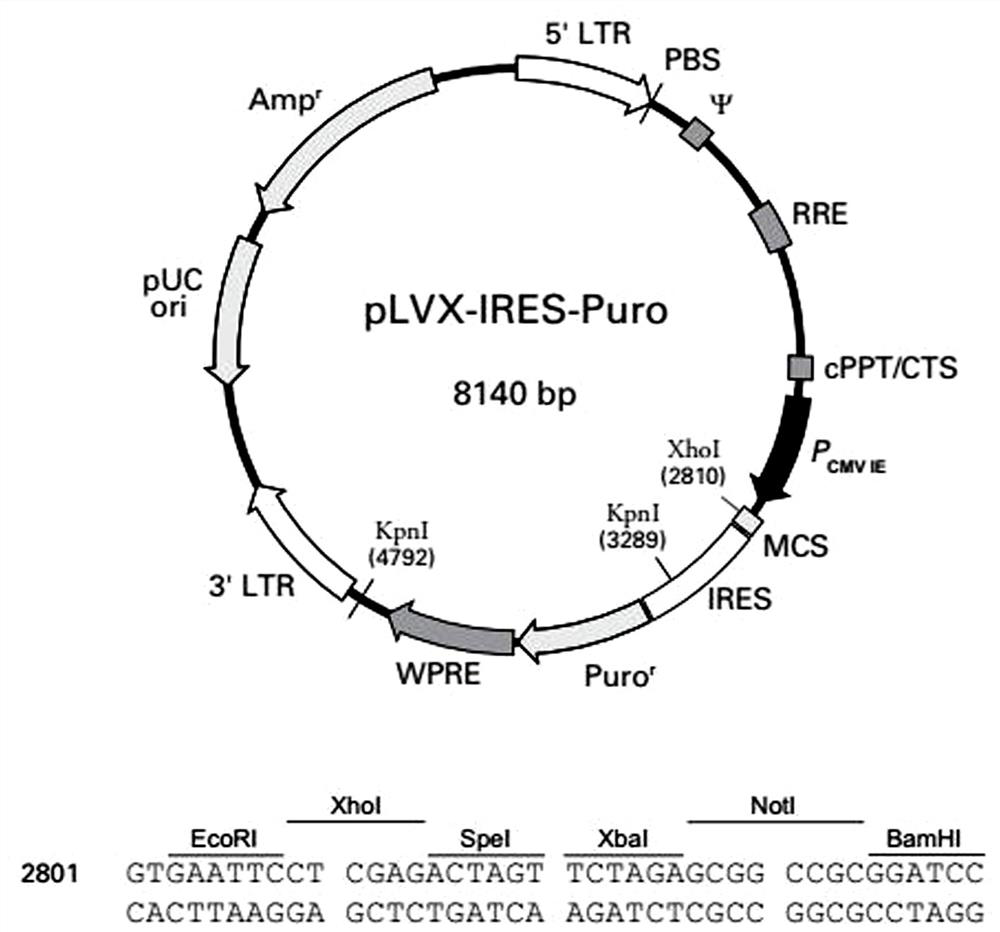
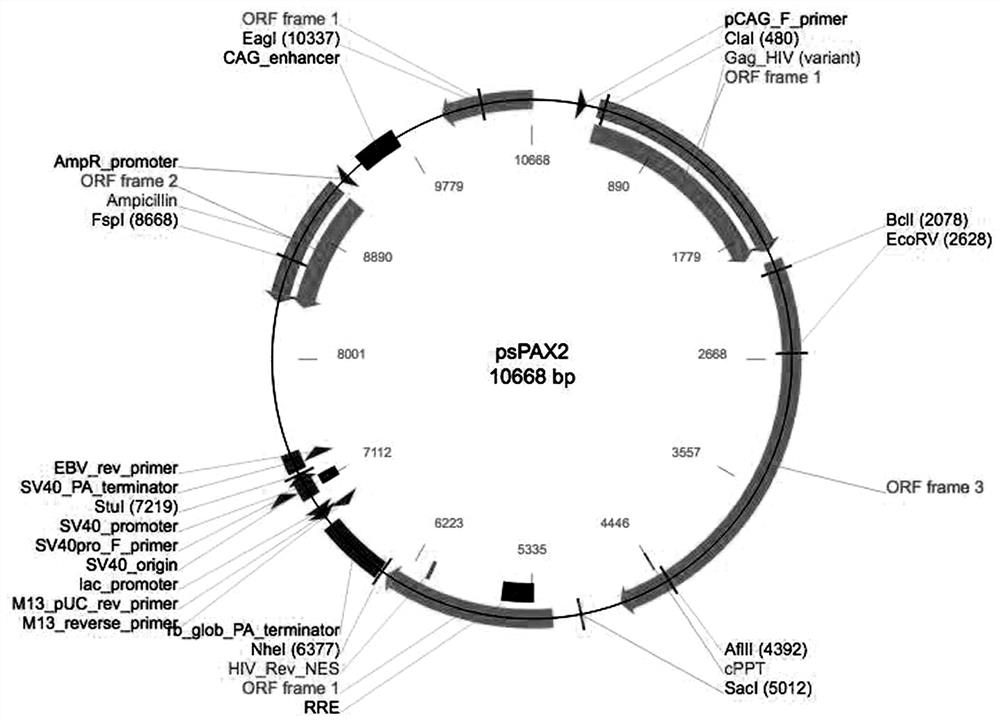
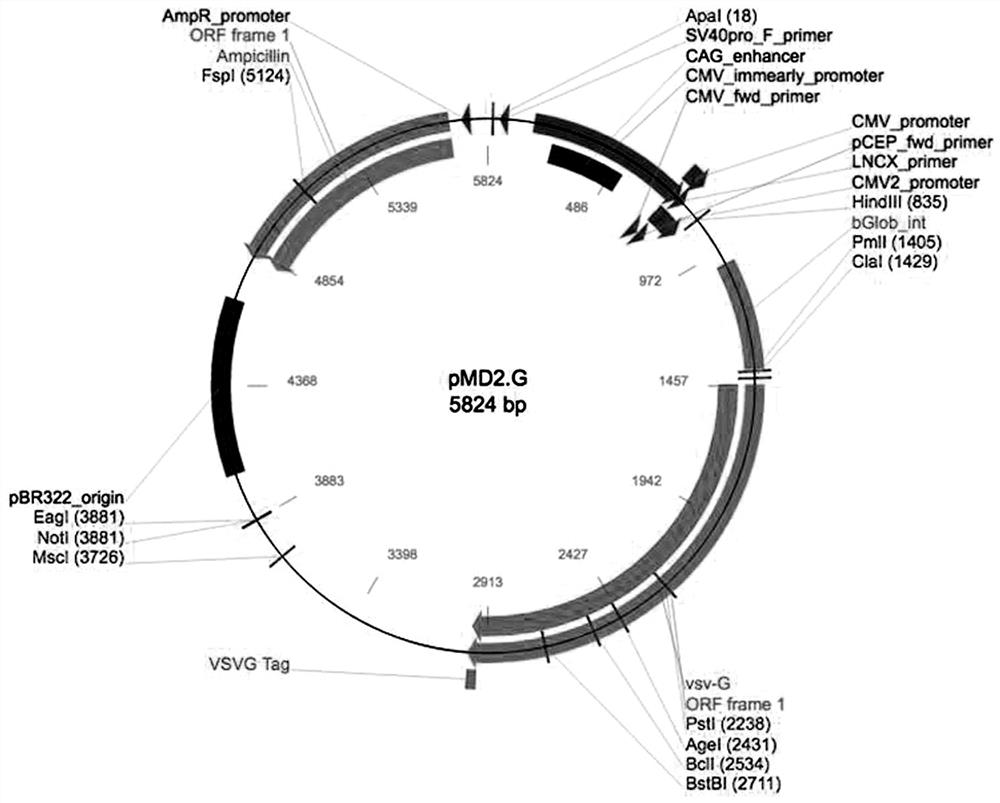
[0021] The lentiviral system includes three plasmids: Plvx-IRES-Puro plasmid expressing reporter gene ( figure 1 ), packaging plasmid psPAX2 ( figure 2 ), envelope protein particle pMD2.G ( image 3 ). Among them, the Plvx-IRES-Puro plasmid contains the puromycin resistance gene and the ampicillin resistance gene, the packaging plasmid psPAX2 contains the gag gene of the HIV virus, which encodes the main structural protein of the virus, and the envelope protein particle pMD2.G contains the herpes simplex The derived VSV-G gene provides the required envelope protein for viral packaging.
[0022] Reporter gene construction: use the known eGFP-COMP fusion gene plasmid as a template, perform polymerase chain reaction to amplify the target fragment (TAKARA company), recover the target fragment from the kit (Shanghai Sangon Bioengineering Co., Ltd.), enzyme The excised fragment and the vector were ligated with T4 DNA Ligase, o...
Embodiment 2
[0024] Embodiment 2: virus transduction HeLa cell
[0025] The collected supernatant contains the recombinant COMP lentivirus, and the packaged virus is used to further transduce HeLa cells, and the stably transfected cell lines are screened. The specific operations are as follows:
[0026] HeLa cells were plated in 6-well plates 24 h before transduction, and when the cells adhered to the wall and grew to about 80%, the old medium was sucked off, and fresh MEM medium (10% fetal bovine serum, 1% Penicillin- Streptomycin) 1.8 mL, recombinant COMP lentivirus 200 μL, and Hexadimethrine bromide (transfection enhancer) 1.6 μL, mixed gently, and cultured in an incubator at 37 °C with 5% CO2;
[0027] After 24 h, the medium containing the virus was sucked off, and 2 mL of fresh medium was replaced, and the culture was continued;
[0028] After 48 h, replace the medium, add 2 mL of fresh medium and add Puromycin (2 μg / mL);
[0029] Afterwards, the medium (containing puro) was changed e...
Embodiment 3
[0031] Example 3: Evaluation of the intracellular COMP content of a cell model using a fluorescence spectrophotometer
[0032] In the stably transfected HeLa cell line, COMP protein and eGFP are fused and expressed, so the content of COMP protein can be indirectly reflected by detecting the content of GFP.
[0033] We used the Cary Eclipse Fluorescence Spectrophotometer (Agilent Technologies) to detect the content of GFP. Initially, we found that GFP can best reflect the relative fluorescence intensity of GFP when the excitation wavelength is 485 nm and the emission wavelength is 530 nm.
[0034] We collected wild-type and mutant cells and cell culture fluid, diluted them with Tris-EDTA solution, and measured the fluorescence intensity of GFP using Cary Eclipse Fluorescence Spectrophotometer (Agilent Technologies). The results were analyzed using the ratio of GFP in cells and GFP in cell culture fluid Ratio to represent changes in intracellular COMP.
[0035] The results show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com