Red organic fluorescence material containing benzothiadiazole derivative as well as preparation method and application of red organic fluorescence material
A technology of benzothiadiazoles and fluorescent materials, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of increased molecular dipole interaction, high radiation transition rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: compound sample preparation
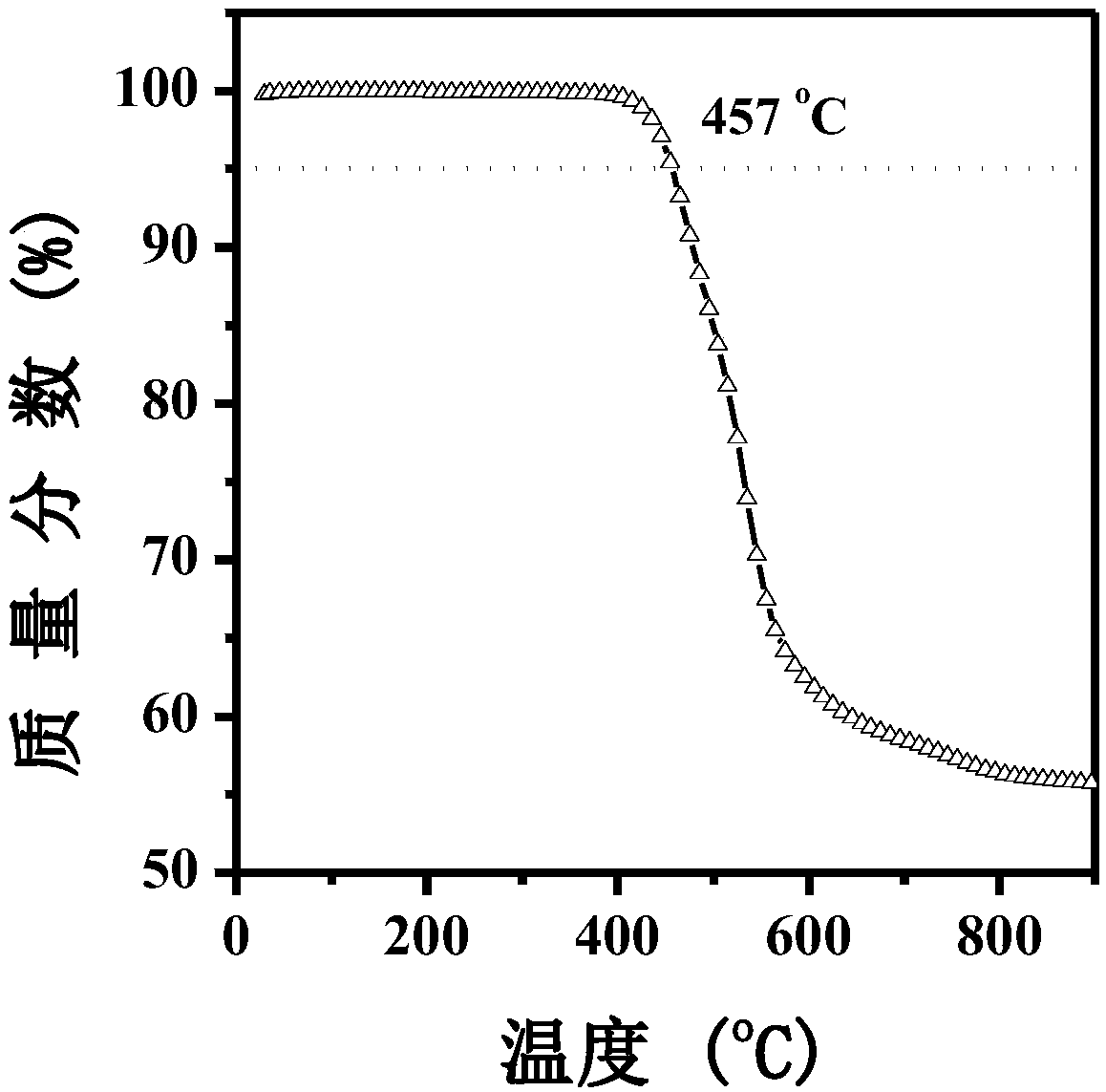
[0068] (1) Weigh 20g (100mmol) 10H-phenothiazine, 31.9g (150mmol) 4-p-bromo-tert-butylbenzene, 14.4g (150mmol) sodium tert-butoxide, 2.9g (10mmol) tri-tert-butyl tetrafluoroborate The base phosphine was added to a 250mL round bottom flask, dissolved in 60mL toluene solvent, and after being vacuumed and nitrogen gas introduced, a catalyst 27.5mg Pd was added to the reaction system. 2 (dba) 3 (3mmol), the reaction was placed in an oil bath at 110°C for 48h. After completion of the reaction, extract with dichloromethane and aqueous phase, take the lower organic layer and carry out dry column chromatography purification, developing agent is sherwood oil, obtain light yellow crystalline powder 40g, i.e. 10-(4-(tert-butyl)benzene Base) phenothiazine, the reaction yield is 90%. 1 H NMR (500MHz, CD 2 Cl 2 )δ7.66(s,1H),7.33(d,J=7.5Hz,1H),7.15–6.77(m,3H),6.24(s,1H),1.54(d,J=20.4Hz,1H), 1.40–1.26(m,1H); MALDI-TOF MS(mass m / z):331.4...
Embodiment 2
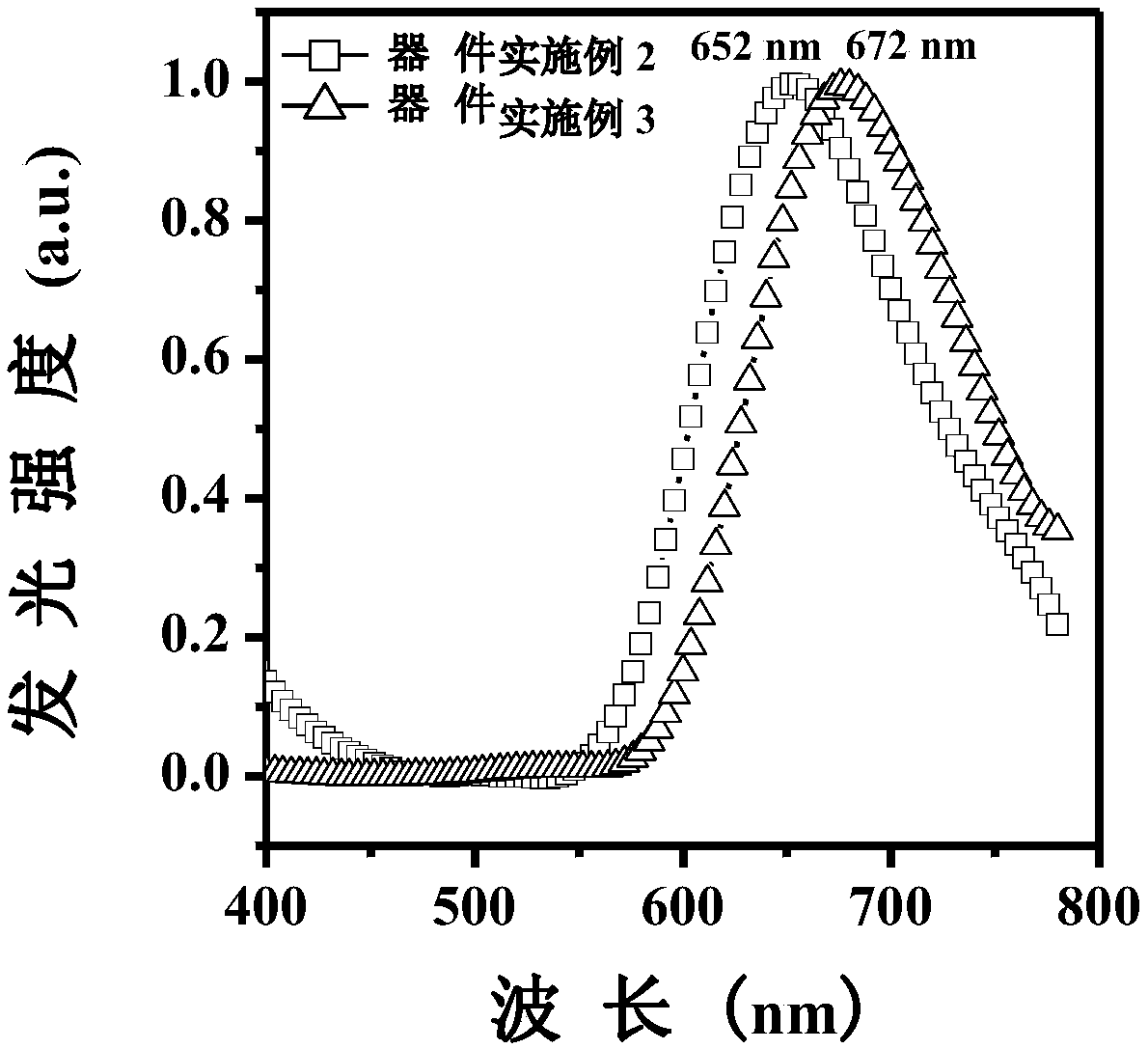
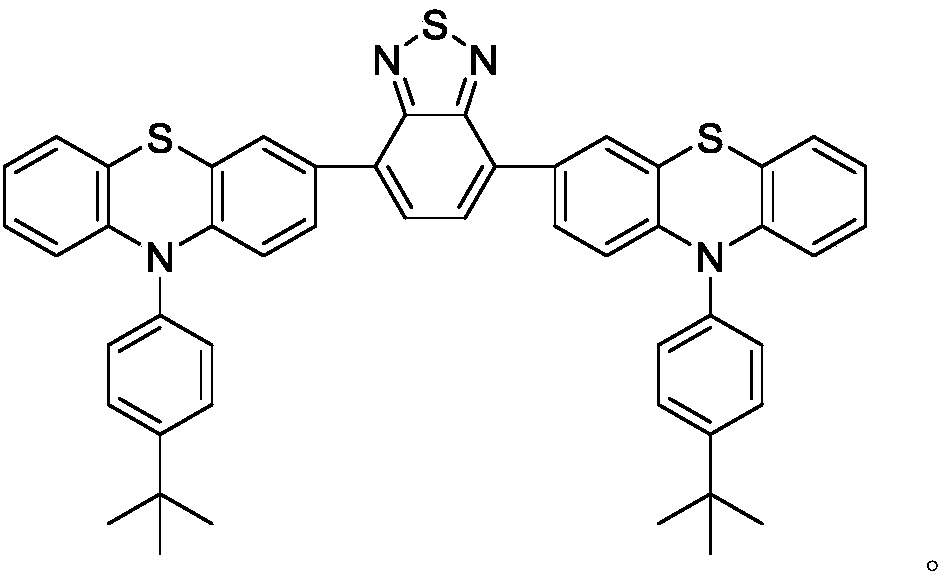
[0074] The device structure is ITO / HATCN(6nm) / NPB(25nm) / TCTA(15nm) / TBPPTZ(20nm) / TPBi(30nm) / LiF(1nm) / Al(100nm), and the device performance results are shown in Table 1.
[0075] Among them, HATCN is the hole injection layer, NPB is the hole transport layer, TBPPTZ is the light-emitting layer prepared by the present invention, TCTA is the electron blocking layer, TPBi is the electron transport layer, and LiF is the electron injection layer.
Embodiment 3
[0077] The device structure is ITO / HATCN(6nm) / NPB(25nm) / TCTA(15nm) / TPBi:TBPPTZ-20%(20nm) / TPBi(30nm) / LiF(1nm) / Al(100nm). The device performance results are shown in Table 1.
[0078] Wherein, HATCN is the hole injection layer, NPB is the hole transport layer, TCTA is the electron blocking layer, TBPPTZ is the guest of the light-emitting layer, and the doping mass percentage is 20%, and TPBi is the matrix of the light-emitting layer, and the doping mass percentage is 80%, TPBi is the electron transport layer, and LiF is the electron injection layer.
[0079] Table 1: Device related parameters
[0080]
[0081] It can be seen from Table 1 that Embodiment 2 and Embodiment 3 have lower driving voltage, higher current efficiency and external quantum efficiency, and have shown good effects on blue light material devices. Using TPBi as the doping matrix material can effectively improve the external quantum efficiency and brightness of the device. The emission peak of the doped de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com