A kind of preparation new method of albendazole
A technology of albendazole and a new method, which is applied in the field of preparation of albendazole, can solve the problems of inability to industrialize production, high equipment investment cost, and high price of propanethiol, and achieve easy industrial production, low content of individual impurities, and safety risks low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of step 1 2-nitro-5-propylthioaniline
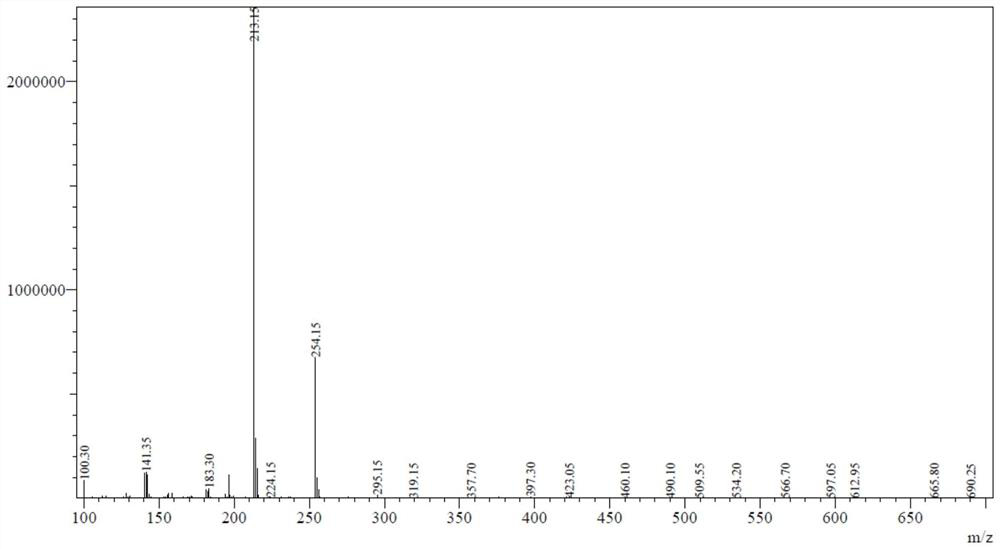
[0049] In a 1L four-necked flask with a thermometer and a stirring device, start stirring, add 172.57g of 2-nitro-5-chloroaniline, ethanol 3 times the weight of 2-nitro-5-chloroaniline, and stir to heat up to 70 -78°C, add potassium sulfide aqueous solution dropwise with 1.2 times the molar weight, and continue to react for 4-5 hours after dropping, add iodo-n-propane with 1.2 times the molar weight of 2-nitro-5-chloroaniline, reflux for 2 hours, and react After cooling to 20°C, 203.14 g of dry product of 2-nitro-5-propylthioaniline was obtained by suction filtration, washing and drying, with a yield of 95.7% and an HPLC content of 98.4%. Mass spectrum data: [M]=213.15 (theoretical molecular weight 212.27)
[0050] Preparation of step 2 4-propylthio o-phenylenediamine
[0051] In a 1L four-necked flask with a thermometer and a stirring device, add ethanol that is 4 times the weight of 2-nitro-5-propylthioaniline,...
Embodiment 2
[0060] The preparation of step 1 2-nitro-5-propylthioaniline
[0061] In a 1L four-necked flask with a thermometer and a stirring device, start stirring, add 172.57g of 2-nitro-5-chloroaniline, methanol with 4 times the weight of 2-nitro-5-chloroaniline, stir and heat up to 60 -65°C, add 2.0 times the molar amount of sodium sulfide aqueous solution dropwise, continue the reaction for 4-5 hours after dropping, add brominated n-propane with 1.5 times the molar amount of 2-nitro-5-chloroaniline, reflux for 2 hours, and react After cooling to 20°C, 203.78 g of dry product of 2-nitro-5-propylthioaniline was obtained by suction filtration, washing and drying, with a yield of 96.0% and an HPLC content of 99.2%.
[0062] Preparation of step 2 4-propylthio o-phenylenediamine
[0063] In a 1L four-necked flask with a thermometer and a stirring device, add 2-nitro-5-propylthioaniline 5 times the weight of isopropanol, 106.14g of 2-nitro-5-propylthioaniline, 2 -Nitro-5-propylthioaniline...
Embodiment 3
[0072] Add 3000ml of reducing and stratified waste water and 30g of sulfur into a 5L reaction bottle, start stirring, raise the temperature to 90-100°C, keep stirring for 5 hours, add 12g of activated carbon, keep stirring at 80-90°C for 1 hour, suction filter, add 20g of chlorine to the filtrate manganese, turn on compressed air bubbling, control the temperature at 70-85°C until the lead acetate test paper is no longer black, filter with suction, concentrate the filtrate to a specific gravity of 1.58mg / ml, slowly cool down to 25°C, and filter with suction, the filter cake is 30- Air-dried at 40°C to obtain 385 g of white sodium thiosulfate with a titration content of 98.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com