Neurored mutant protein and preparation method thereof
A mutant and protein technology, applied in the field of neuroglobin mutant protein and its preparation, can solve the problem of not improving the stability of neuroglobin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Based on genetic engineering and protein engineering, using site-directed mutagenesis technology, cysteine (Cys) was introduced into the 15th position of neuroglobin, and the mutant plasmid was expressed in E. coli BL21 (DE3), and 176g of bacteria were broken by 60W ultrasonic for 1h, followed by 176g / L, 195g / L ammonium sulfate salting-out dialysis and DEAE anion exchange, column separation G75, cation exchange column MonoQ method, to separate and purify protein, and obtain A15C Mb mutant protein.
[0031] The amino acid sequence (SEQ ID NO.1) of A15C Mb is as follows:
[0032] MERPEPELIRQSWRCVSRSPLEHGTVLFARLFALEPDLLPLFQYNCRQFSSPEDCLSSPEFLDHIRKVMLVIDAAVTNVEDLSSLEEYLASLGRKHRAVGVKLSSFSTVGESLLYMLEKCLGPAFTPATRAAWSQLYGAVVQAMSRGWDGE
Embodiment 2
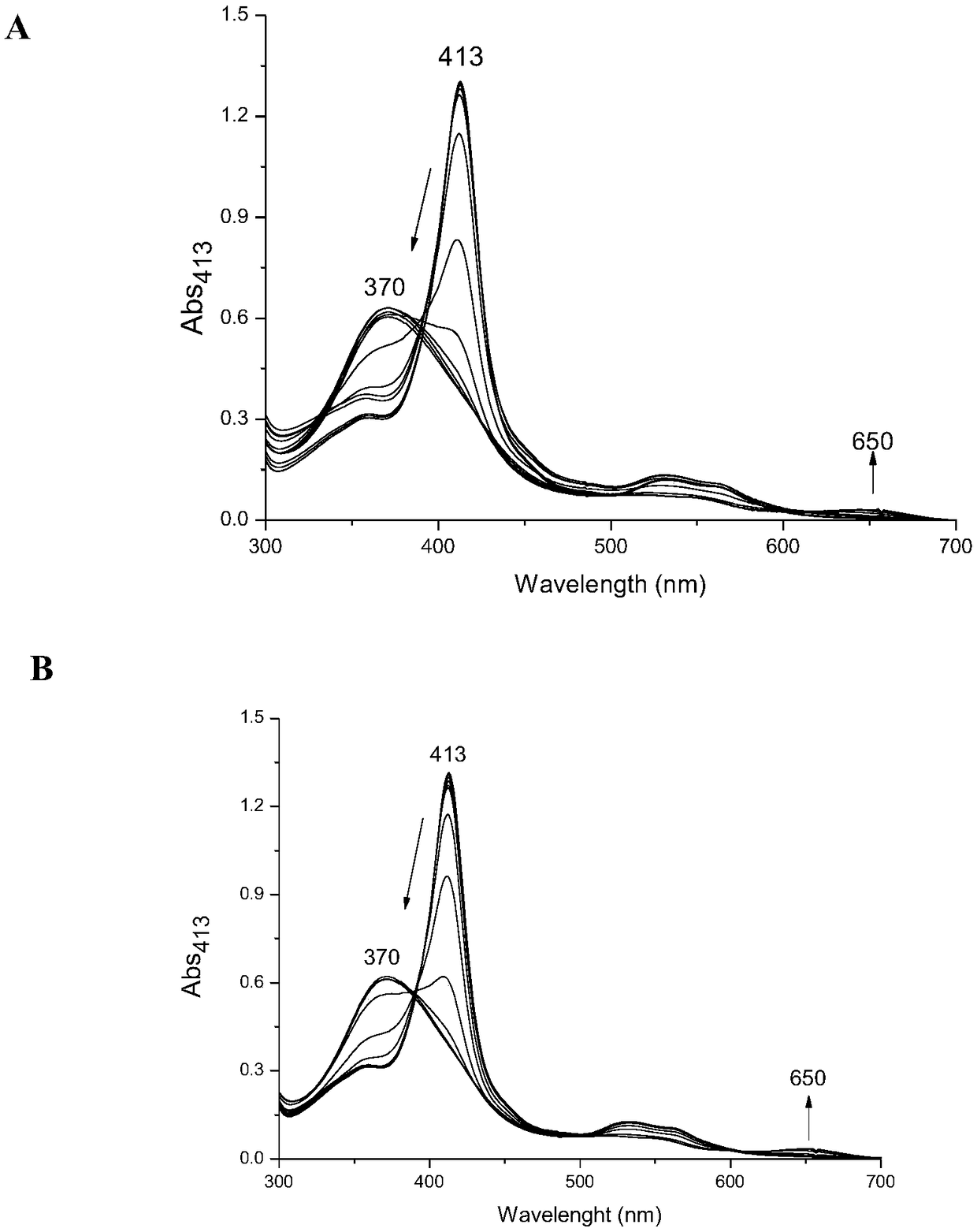
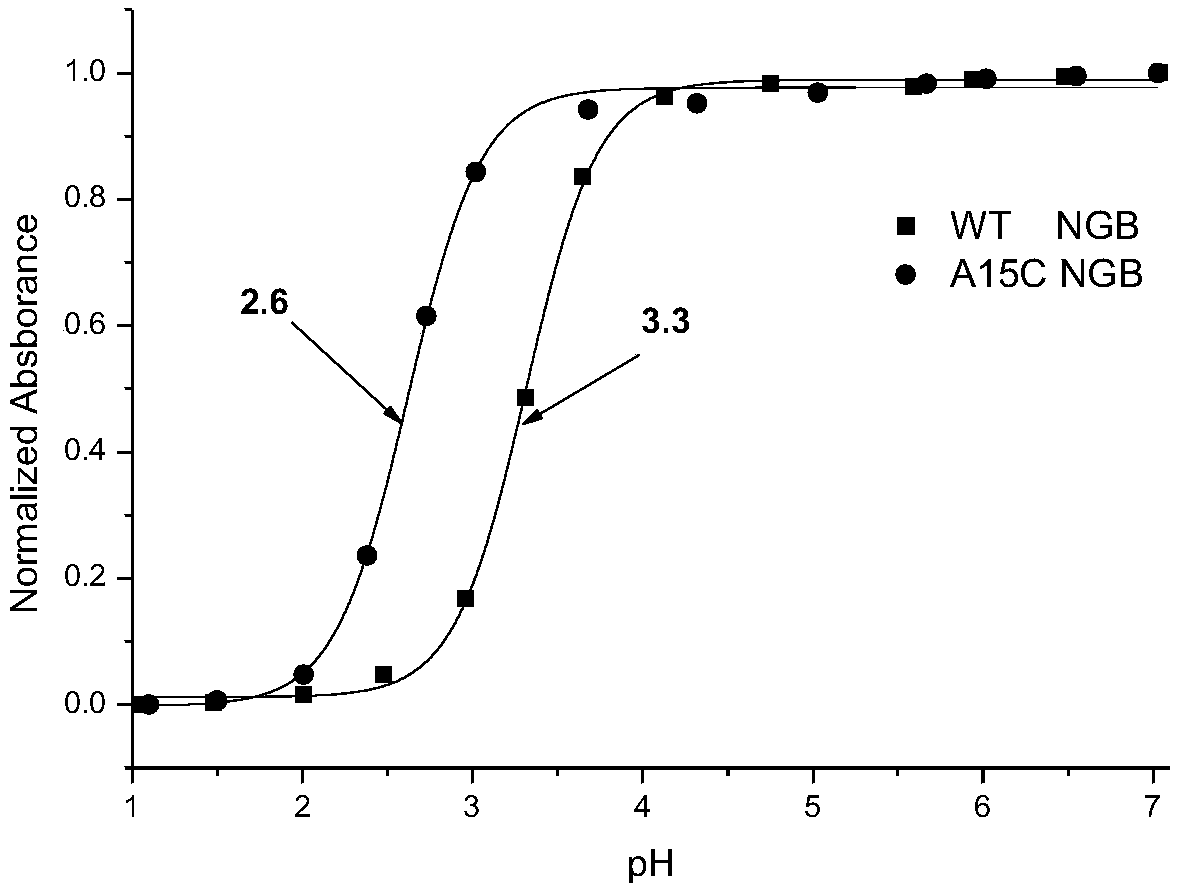
[0034] The WT Ngb (wild-type neuroglobin) and A15C Ngb proteins were respectively dissolved in a potassium dihydrogen phosphate-potassium hydroxide buffer solution (pH 7.0) so that the concentration of the protein solution was 10 μM. Add a small amount of high-concentration hydrochloric acid solution to the two protein solutions several times to lower the pH value step by step, record the protein UV-visible spectra corresponding to each pH value, and obtain the corresponding full spectrum ( figure 1 ) and the UV absorbance value at 413nm of the soret peak (A 413nm ) changes with the decrease of pH value ( figure 2 ). Through equation fitting, the denaturation midpoint of WT Ngb was pH 3.3, while that of A15C Ngb was pH 2.6, which indicated that the mutant protein A15C was more stable.
Embodiment 3
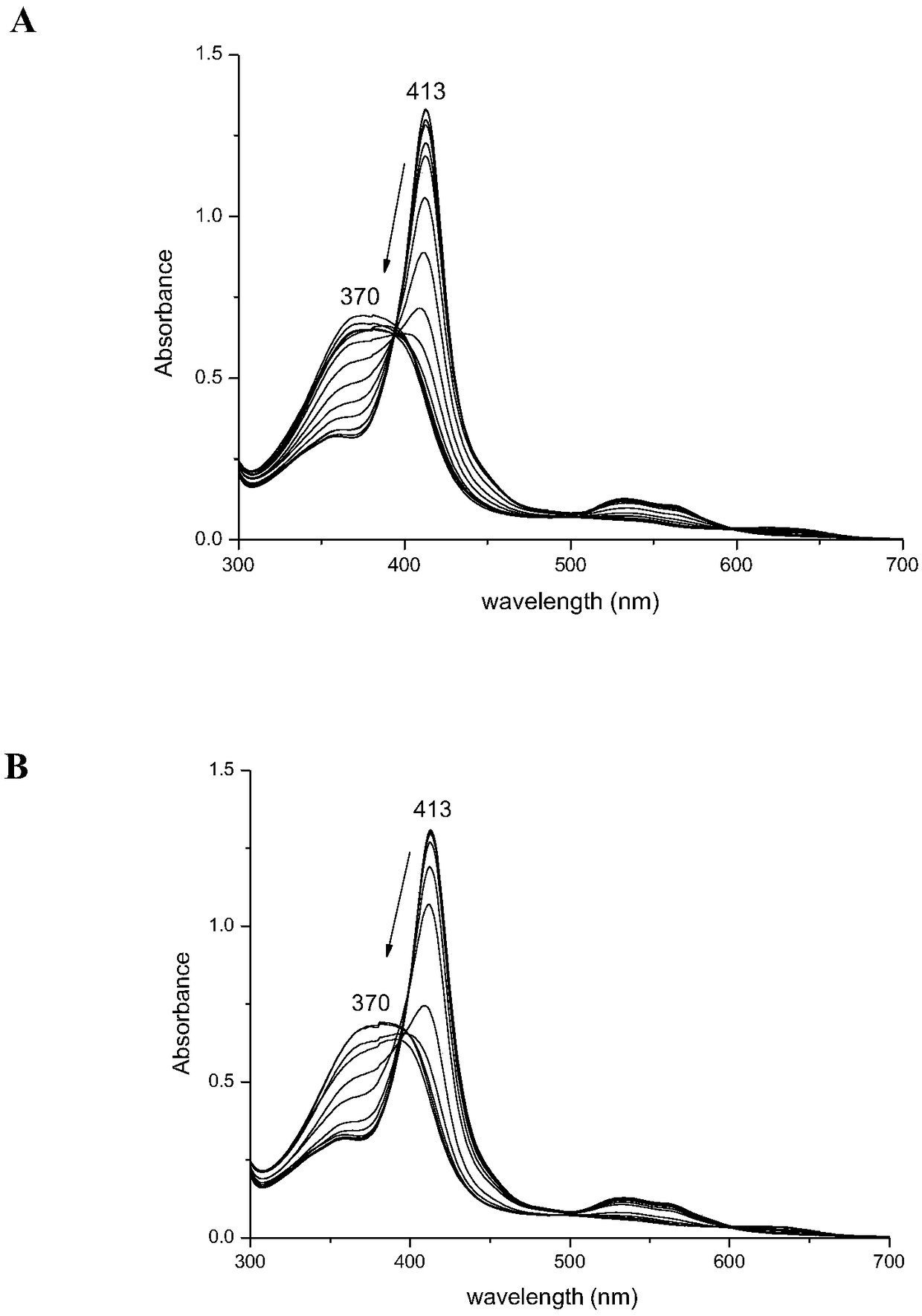
[0036] Prepare 200 μL of WT Ngb protein and A15C Ngb protein at a concentration of 2 mM, respectively, and add 10 μL of 2 mM WTNgb protein and A15C Ngb protein samples into 2 mL of a certain gradient concentration of guanidine hydrochloride solution (0-6.0 M) and mix well. Under the condition of 25°C, after mixing evenly, let it stand for 30 minutes, then measure the ultraviolet spectrum of the protein under different guanidine hydrochloride concentrations in sequence, and record the change of the whole spectrum ( image 3 ). The obtained spectral data is plotted by Origin software, with different guanidine hydrochloride concentrations as the abscissa, and the protein Soret peak absorbance value as the ordinate, and the Boltzmann equation is used to fit the guanidine hydrochloride concentration at the midpoint of denaturation, and the processing is normalized. Figure ( Figure 4). Through equation fitting, the midpoint of denaturation of WT Ngb corresponds to a concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com