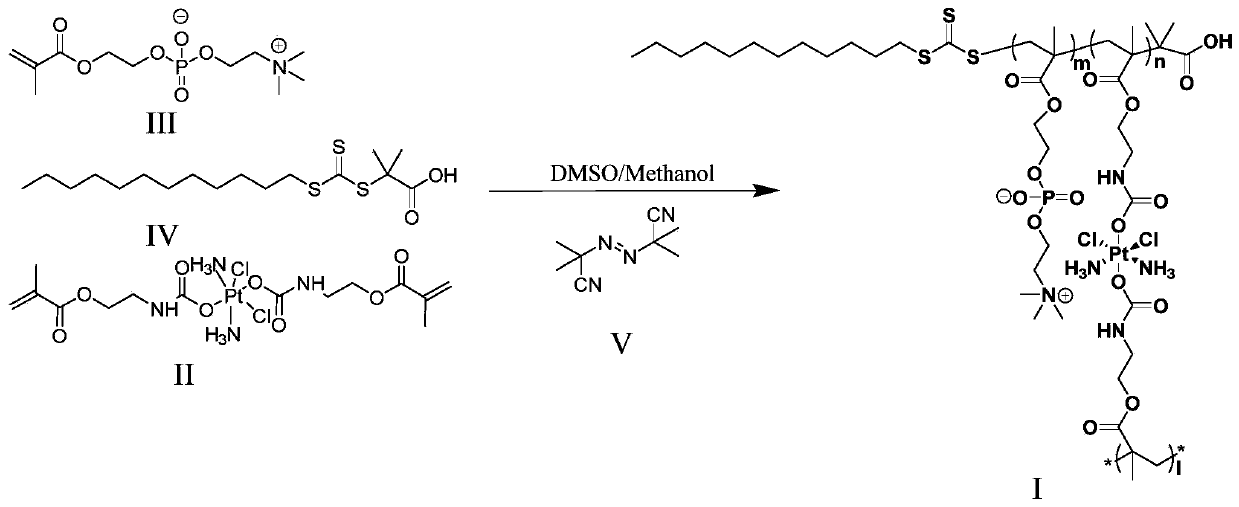
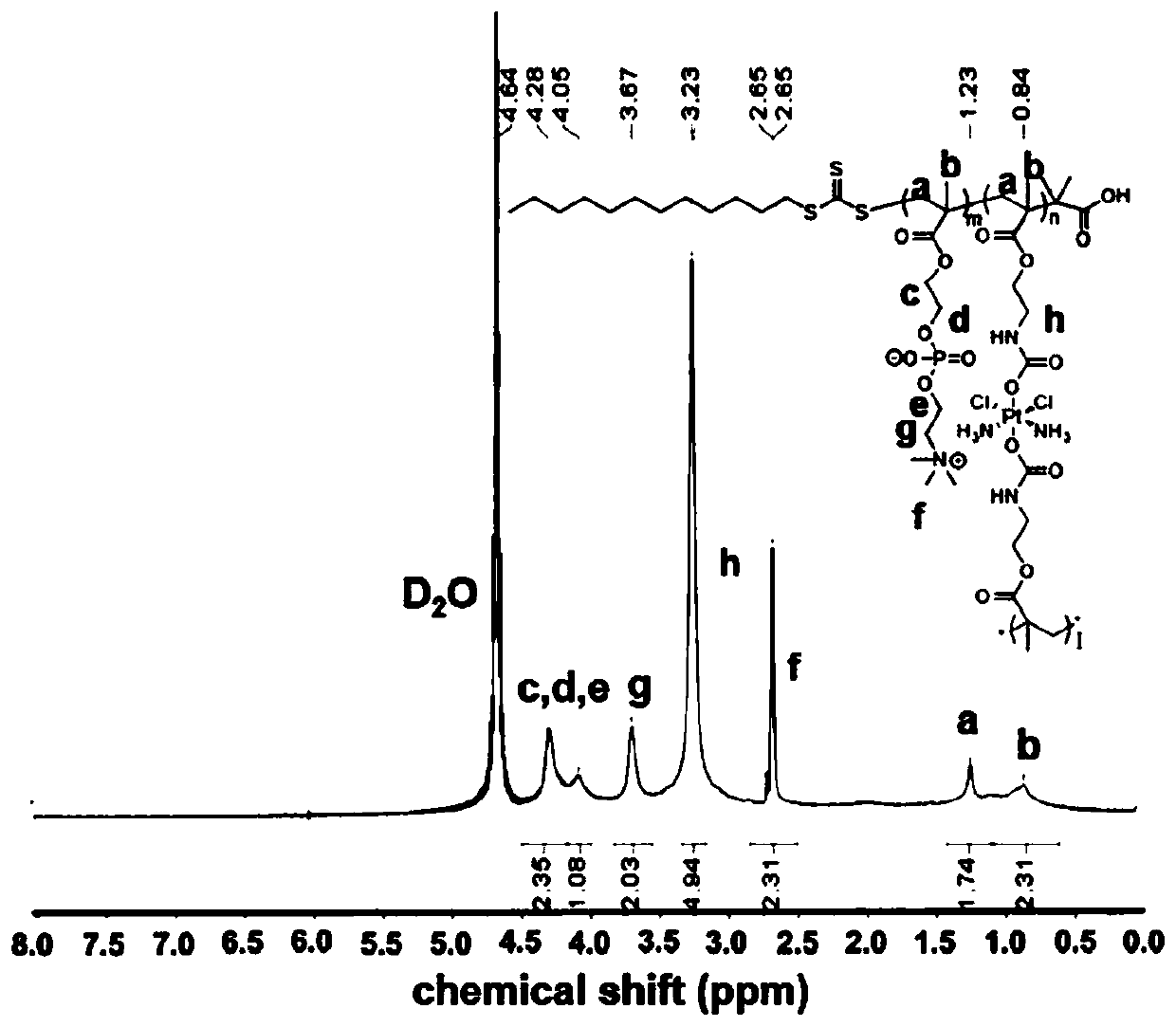
Use of tetravalent cisplatin prodrugs in combination with bioreductive drugs
A cisplatin and prodrug technology, applied in the field of combined use of tetravalent cisplatin prodrugs and bioreductive drugs, can solve problems such as poor effect, and achieve the effects of single and stable size, high yield, and improved curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of hyperbranched platinum prodrugs. Get 168mg of cis-dichlorodiamine dimethyl diacetamido methyl methacrylate platinum (IV) and 283.2mg and add 2-methacryloyloxyethyl phosphorylcholine to a 50mL eggplant type reaction flask, then 9.1 mg of RAFT chain transfer agent 2-(dodecyltrithiocarbonate)-2-isobutyric acid and 1 mg of initiator azobisisobutyronitrile were sequentially added, followed by 10 mL of anhydrous dimethylmethylene The mixed solvent of sulfone / anhydrous methanol was protected by nitrogen gas, and the stirring was started, followed by three freezing-pumping-thawing processes, the reaction time was 20 hours in the dark, and the reaction temperature was 60-70°C. After the reaction was completed, cool down to room temperature, absorb the reaction product and drop it into 200mL of cold ether, a precipitate appeared and allowed to stand for 2 hours, then the precipitate was collected by centrifugation, and a light yellow solid was obtained after drying. ...
Embodiment 2
[0054] Synthesis of hydrogel-type tetravalent platinum prodrugs. Take 26.88 mg of cis-dichlorodiamine dimethyl diacetamidomethyl methacrylate platinum (IV) and 283.2 mg of 2-methacryloyloxyethyl phosphorylcholine into a 50 mL eggplant-shaped reaction bottle, Then 9.1 mg of RAFT chain transfer agent 2-(dodecyltrithiocarbonate)-2-isobutyric acid and 1 mg of initiator azobisisobutyronitrile were sequentially added, followed by 10 mL of anhydrous dimethyl The mixed solvent of sulfoxide / anhydrous methanol was protected by nitrogen, and the stirring was started, followed by three freezing-pumping-thawing processes, the reaction time was 20 hours in the dark, and the reaction temperature was 60-70°C. After the reaction was completed, cool down to room temperature, absorb the reaction product and drop it into 200mL of cold ether, a precipitate appeared and allowed to stand for 2 hours, then the precipitate was collected by centrifugation, and a light yellow solid was obtained after dr...
Embodiment 3
[0059] This embodiment relates to a preparation method of platinum-based prodrug-loaded hypoxic drug tirapazamine, which includes the following specific steps:
[0060] Dissolve 1-10 mg of tirapazamine in 1-10 mL of platinum prodrug with a concentration of 1-10 mg / mL, stir in the dark for 1-24 hours, dialyze, and the loaded tirapazamine content is determined by using UV / Vis spectroscopy It is obtained by measuring the absorption at 500nm with a luminance meter.
[0061] Figure 4 is the in vitro release curve of the cisplatin long-circulation nanogel prepared in Example 3. In vitro release assay method: Precisely pipette 2 mL of freshly prepared purified cisplatin liposomes into a dialysis bag (30KD). Put into 20mL release medium, sample 1mL at 0, 1, 2, 4, 8, 10, 18, 30, 42, and 50 hours respectively, and supplement 1mL of fresh medium at the same time, the test of ICP-AES is used to test the Pt content, using ultraviolet / Visible spectrophotometer test wavelength at 500nm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com