Phospholipase B and application in preparing glycerolphosphocholin thereof
A technology of glycerol phosphatidylcholine and phosphatase, which is applied in hydrolytic enzymes, microbial-based methods, biochemical equipment and methods, etc., can solve the problems of high price, limited sources of animal and plant phospholipase B, and improve catalytic efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
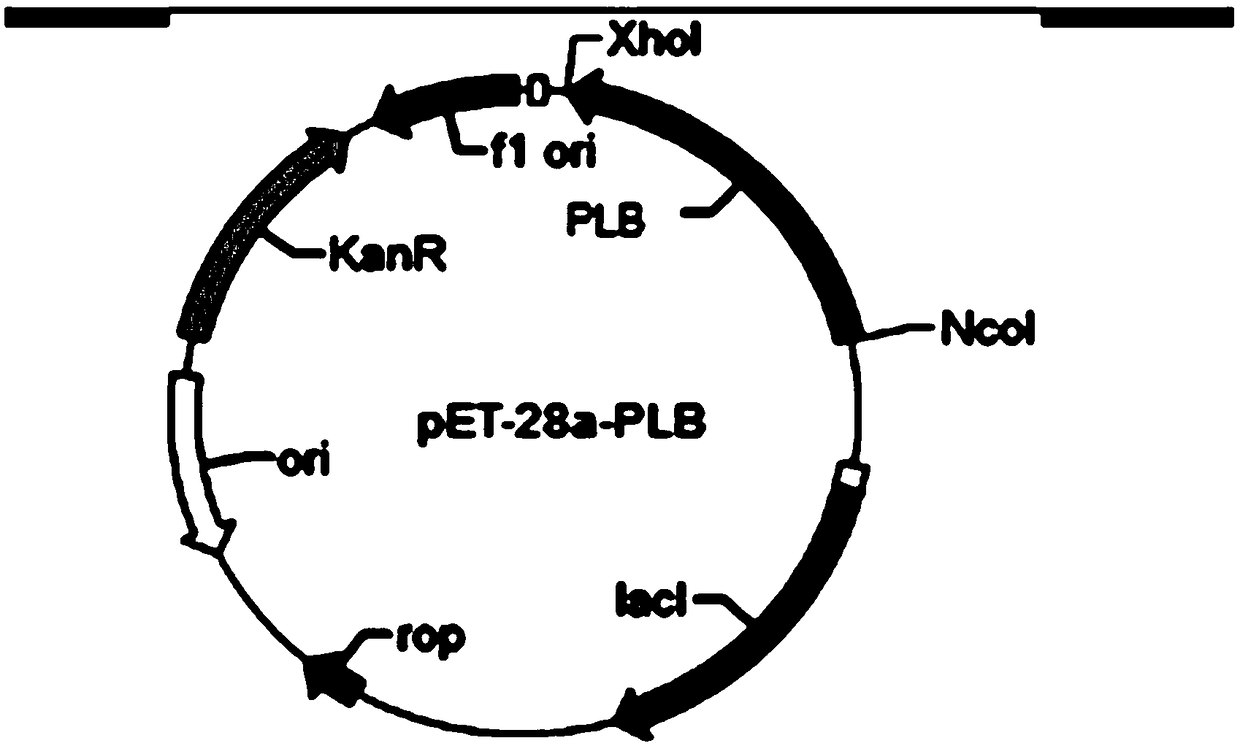
[0027] Example 1 Construction of recombinant Escherichia coli BL21(DE3)-pET28a-plb
[0028] According to the reported Pse μdomonas fl μorescens The amino acid sequence of the source phospholipase B (NCBI accession number: GM367870) was codon-optimized and synthesized. The codon-optimized sequence is shown in SEQ ID NO.1, and restriction sites NcoI and XhoI were designed at both ends.
[0029] Upstream primer P1: (CATGCCATGGGCATGAAAAAAGTTATGCTG)
[0030] Downstream primer P2: (CCGCTCGAGGAAACGGTAGGTAGC), subcloned into vector PET-28a to obtain recombinant plasmid pET-28a-PLB. The constructed recombinant plasmid PET-28a-PLB was transformed into Escherichia coli expression host BL21(DE3) to obtain phospholipase B expression strain BL21(DE3)pET-28a-PLB.
Embodiment 2
[0031] Example 2 Culture and expression of Escherichia coli BL21(DE3)-pET28a-plb
[0032] Pick a single colony of genetically engineered bacteria expressing phospholipase B, inoculate it in LB medium, and culture it overnight at 37°C; then transfer the bacteria to LB medium, the inoculation amount is 1-5% of the volume of LB medium, Cultivate at 37°C for 2-3 hours to OD 0.4-0.6, add IPTG to a final concentration of 0.5mmol / L, culture overnight at 30°C, collect the genetically engineered bacteria expressing phospholipase B by centrifugation, and perform cell disruption at 8000rpm under the condition of centrifugation The supernatant enzyme solution was collected for 20 minutes, and then the enzyme activity was detected.
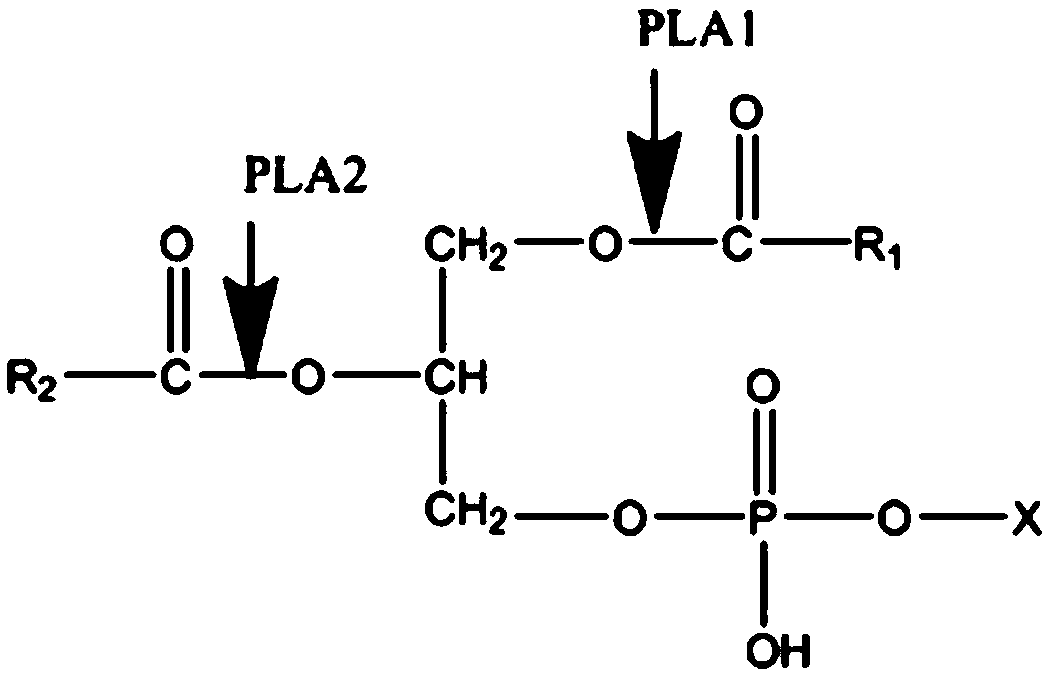
[0033] Detection method for enzyme activity detection: add 100µL enzyme solution diluted in a certain proportion to 5mL PBS buffer solution (pH 7.0) containing egg yolk lecithin, so that the final concentration of egg yolk lecithin is 60g / L, mix well and place...
Embodiment 3
[0037] Example 3 Research on protein expression of phospholipase B expression strain
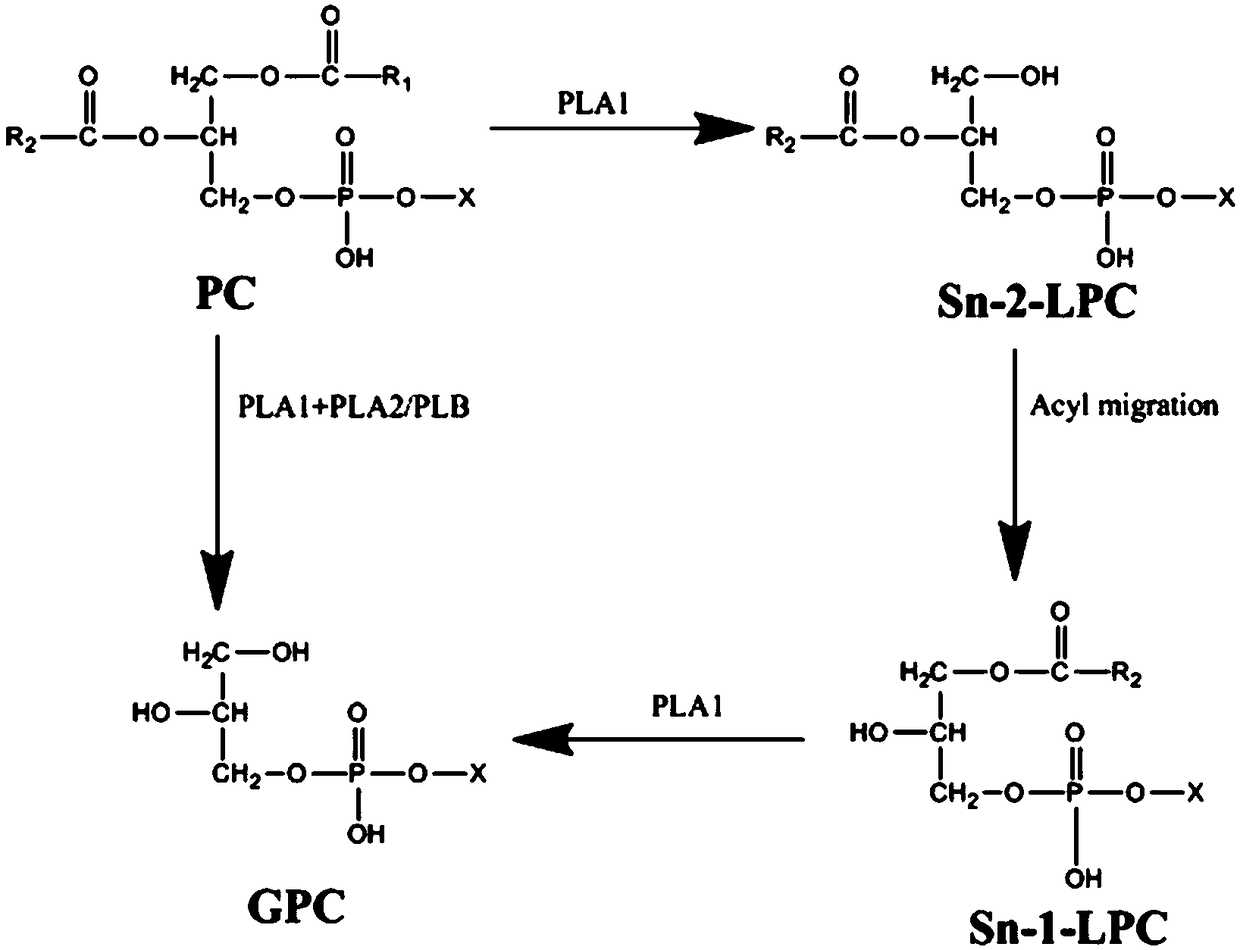
[0038]Pick a single colony of genetically engineered bacteria expressing phospholipase B, inoculate it in 5 mL of LB medium, add 50 mg / mL of kanamycin, and culture it at 37°C for 6-8 hours; then transfer the bacteria to LB medium, inoculate The amount is 1-5% of the volume of LB medium, add 50mg / mL kanamycin, incubate at 37°C for 2-3 hours to OD 0.4-0.6, add IPTG to a final concentration of 0.5mmol / L, incubate at 30°C for 12h , collect the genetically engineered bacteria expressing phospholipase B by centrifugation, break the cells, and centrifuge at 8000 rpm for 20 minutes to collect the supernatant enzyme solution, and perform protein electrophoresis on the enzyme solution and the crushed precipitate to verify the protein expression of phospholipase B. At 30°C, under the induction condition of IPTG0.5mmol / L, the study found that there were more serious inclusion bodies (such as image 3 )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com