Preparation method of soluble recombinant proteins
A recombinant protein, soluble technology, applied in the field of preparation of soluble recombinant protein, can solve the problems of insoluble and inactive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0023] Example 1.1 Cloning of human IL-6 gene:
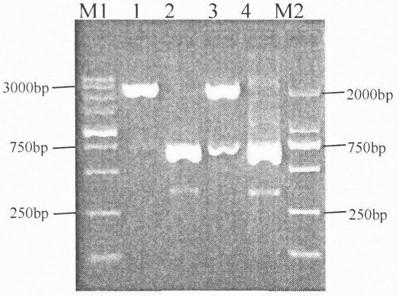
[0024] Method: According to the gene bank published IL-6 gene sequence (serial number: NM00060), use PrimerPremier5.0 software to design a pair of primers P1: 5'ACG GAATTC CCAGTACCCCCCAGGAGAAG 3', the underlined part is the enzyme cutting site EcoRI, P2: 5'AATAG CTCGAG TTACATTTGCCGAAGAGCC 3', the underlined part is the XhoI restriction site. The total mRNA extracted from human peripheral blood lymphocytes was used as a template, and it was first reverse-transcribed into cDNA, and then the cDNA was used as a template to amplify the complete human recombinant IL-6 gene fragment with IL-6 specific primers, and the recovered PCR product was connected to PTG19 -T vector, the plasmid PTG-IL-6 was obtained, which was confirmed to be the correct sequence by sequencing, and the identification results are shown in figure 1 ;
Embodiment 12
[0025] Example 1.2 Prokaryotic expression vector construction:
[0026] 1. Use a DNA recovery kit to recover the PCR product.
[0027] 2. Digest the recovered PCR amplification product with restriction endonuclease with EcoRI and XhoI to obtain the digested product;
[0028] 3. Digest the expression vector pET-32a(+) with restriction endonucleases EcoRI and XhoI, and use the DNA recovery kit to recover the vector skeleton;
[0029] 4. Ligate the digestion product of step 2 and the vector backbone of step 3 with T4 DNA ligase at 4°C overnight to obtain the ligation product;
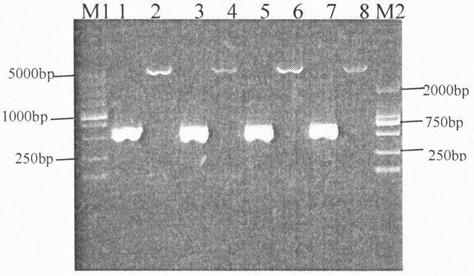
[0030] 5. Transform the ligation product into Escherichia coli DH5a competent cells, and pick a single colony for colony PCR and enzyme digestion identification: 1) Colony PCR uses primers P1 and P2, and the result is that a colony with a clear single band at about 570bp is a positive colony ; 2) The enzymes used for enzyme digestion identification are restriction endonucleases EcoRI and XhoI, and the co...
Embodiment 13
[0032] Example 1.3 Induced expression of recombinant interleukin 6 and optimization of expression conditions
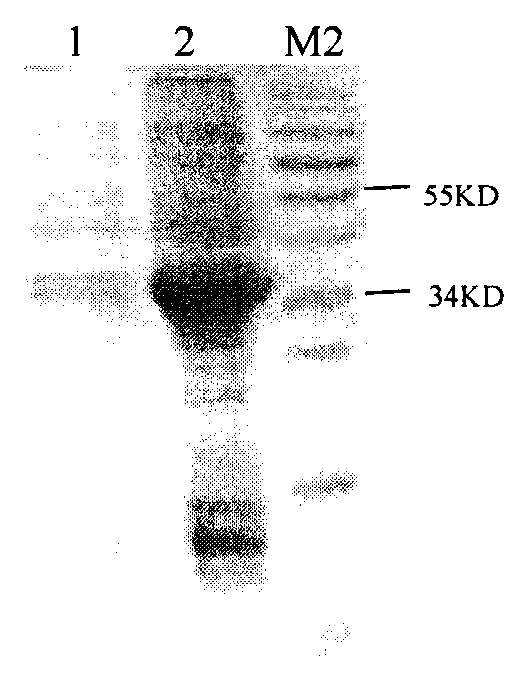
[0033]1. Inoculate the recombinant engineered bacteria 141 in 5 mL of liquid LB medium containing ampicillin resistance (Amp+), and culture it on a shaker at 37° C. at 200 rpm until the OD600 value is 0.8, then add the inducer IPTG (isopropyl Thiogalactoside, final concentration 0.5mM / L), 180rpm continued to induce culture, and the induction time was 5 hours: a control group was set up at the same time, except that the inducer IPTG was not added, other operations were the same, and the recombinant protein was detected by SDS-PAGE electrophoresis. protein expression, see image 3 ;
[0034] 2 Resuscitate the prepared 141 engineering bacteria and set aside; inoculate the fresh cultures into 5 mL of LB liquid medium containing Amp, shake and culture at 37°C for 3 hours, and adjust the final concentrations of 0.02, 0.04, 0.06, 0.08, Add IPTG at 0.1 and 0.2mM, continue t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com