Steroidal 11β-hydroxylase from Curvularia lunae and its coding gene and application
A technology for encoding and transgenic cell line, applied in steroid 11β-hydroxylase and its encoding gene and application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
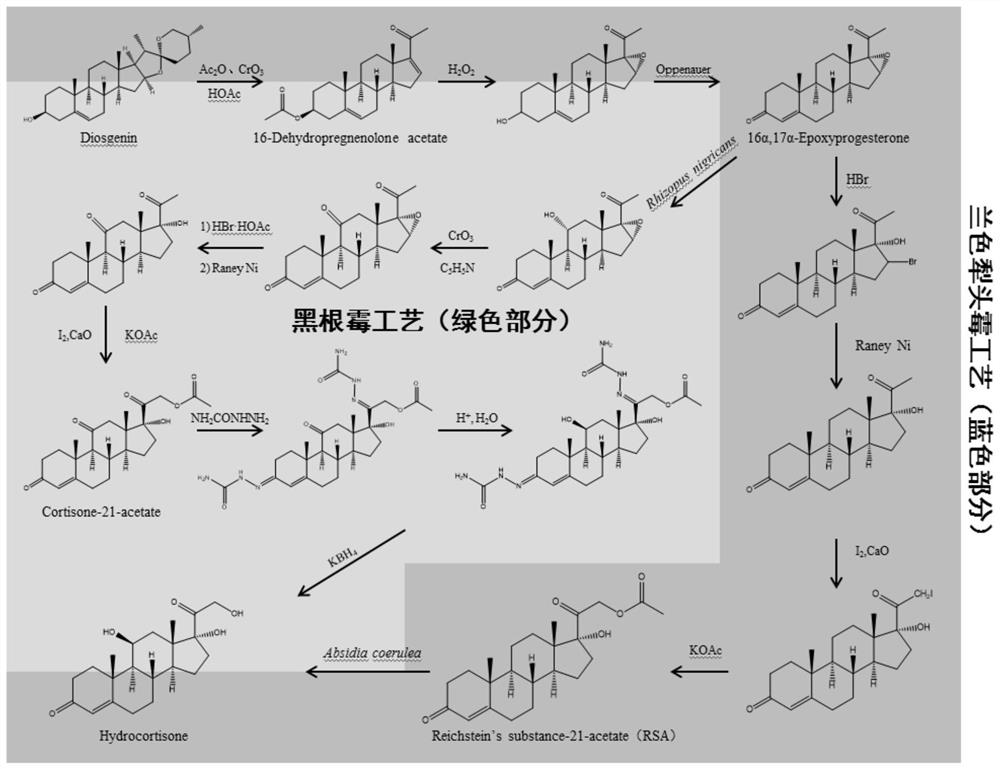
[0085] Example 1. Cloning and expression of β-hydroxylase at position 11 of Campylobacter crescentus
[0086] The cloning and expression of the gene is divided into the following three steps:
[0087] 1. Extraction of total RNA from Campylobacter crescentus
[0088] First, Campylobacter crescentus AS3.4381 (ATCC12017) was cultured on a plate for several days, and a certain number of spores were collected and inserted into 50 mL potato-glucose (PDA) medium, and cultured overnight to synthesize a large number of cells; then , centrifuged to collect the mycelium of Campylobacter crescentus, washed with potassium phosphate buffer (PBS), and finally resuspended with 50 mL of buffer and added the substrate hydrocortisone 21-acetate with a final concentration of 170 mg / L ( RSA) was induced for 2 h, and samples were taken for RNA extraction.
[0089] RNA extraction method:
[0090] (1) Add 0.5mm grinding beads (to fill the bottom of the conical tube) and 1 mL of Trizol to a 2.0 mL ...
Embodiment 2
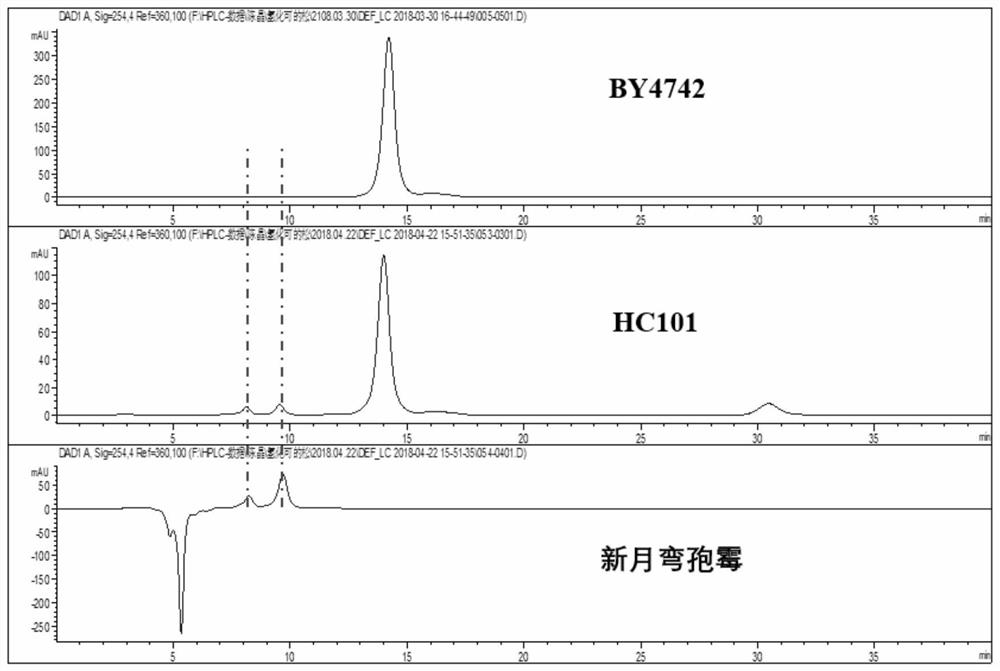
[0116] Example 2. Construction of Saccharomyces cerevisiae engineering strain HC101
[0117] The starting bacterium Saccharomyces cerevisiae BY4742 was grown overnight in selection medium. The composition of the liquid screening medium is as follows: SD-Trp (Beijing Pankino (Functional Genome) Technology Co., Ltd.), 2% glucose, 0.005% His., 0.01% Leu., 0.01% Ura. 100mL). Take 1ml (OD about 0.6-1.0) into 1.5ml EP tubes, centrifuge at 10000g at 4°C for 1 min, discard the supernatant, wash the precipitate with sterile water (4°C), centrifuge under the same conditions, and discard the supernatant. 1 ml of treatment solution (10 mM LiAc; 10 mM DTT; 0.6 M sorbitol; 10 mM Tris-HCl (pH 7.5) was added to the bacterial cells, and DTT was added only when the treatment solution was used), and placed at 25°C for 20 min. Centrifuge, discard the supernatant, add 1ml of 1M sorbitol (0.22μm aqueous membrane sterilization) to the cells to resuspend, centrifuge, discard the supernatant (resusp...
Embodiment 3
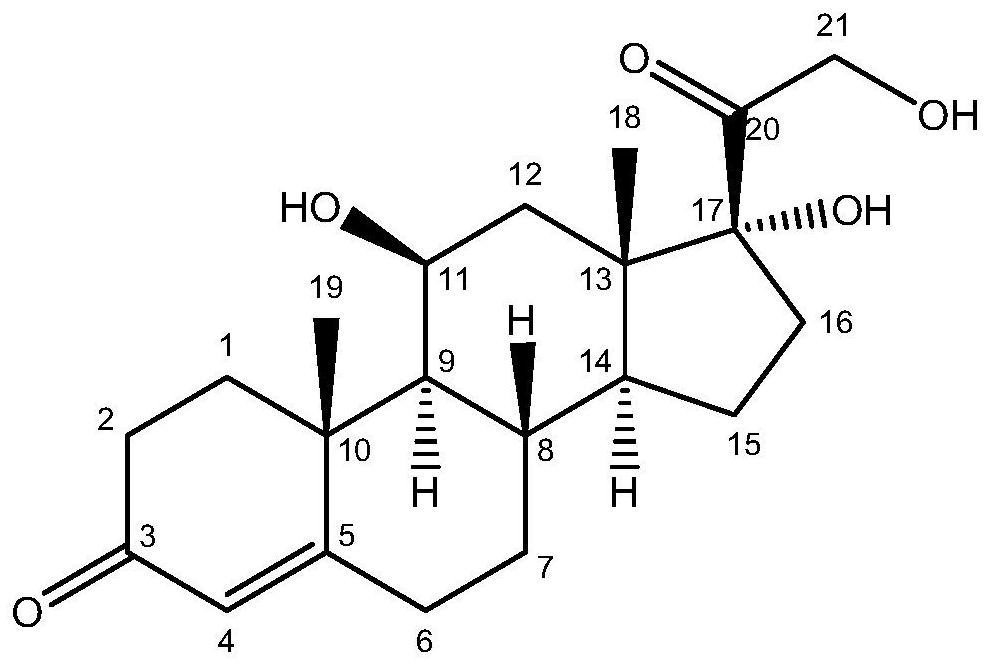
[0118] Example 3. Catalytic synthesis of hydrocortisone and 14α-hydroxycortisol by Saccharomyces cerevisiae engineering bacteria HC101
[0119] Shake flask fermentation catalysis: in solid selective medium (recipe: solid yeast screening medium SD-Ura-Trp, 2% glucose, 0.005% His, 0.01% Leu, 1.5% agar; each percentage sign represents g / 100mL) Activated HC101 yeast strain in the corresponding liquid selection medium (recipe: liquid yeast screening medium SD-Ura-Trp, 2% glucose, 0.005% His, 0.01% Leu; each percentage sign indicates g / 100mL) The seed solution (30°C, 250rpm, 16h) was inoculated into three 500mL conical flasks containing 100mL YPD liquid medium with an inoculum volume of 1mL, 30°C, 250rpm shaking culture for 2 days, 5000rpm to collect yeast cells, and buffered with PBS The solution was washed and finally resuspended in a 250 mL Erlenmeyer flask containing 30 mL of PBS, and the substrate RSA with a final concentration of 170 mg / L was added to conduct a catalytic react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com