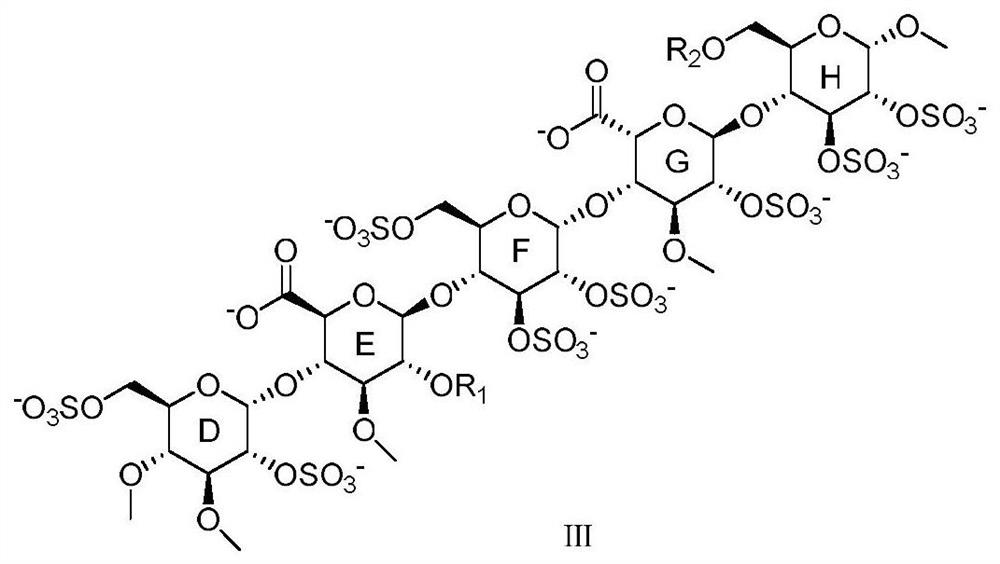
Anticoagulant pentasaccharide compound, preparation method and medical use thereof
A compound and composition technology, applied in the field of pentasaccharide compounds, can solve problems such as increased bleeding risk, and achieve the effects of prolonging the half-life in vivo, strong anticoagulant factor Xa activity, and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] Preparation of Compound D1: Dissolve D0 (748g) in tetrahydrofuran, use excess potassium hydroxide as a base, add dimethyl sulfate dropwise at 0°C for 2 hours, add potassium hydroxide aqueous solution to quench for 4 hours after the reaction, and then extract the fraction with ethyl acetate liquid, spin-dried to obtain D1 (804g, yield 100%).
[0070] Preparation of compound D2: Add D1 (804g) to acetic acid: water: sulfuric acid = 2000g:400g:98g, reflux reaction for 1h, add ethyl acetate and water for extraction, and evaporate the organic phase to dryness to obtain D2 (690g, yield 89%) .
[0071] Preparation of compound D3: D2 (388g) was dissolved in anhydrous dichloromethane, added with 288g of trichloroacetonitrile and 15g of DBU to react for 1h, and then spin-dried to obtain D3 (489g, yield 92%) by column chromatography.
[0072] The second part preparation of monosaccharide E ring
[0073] synthetic route:
[0074]
[0075] a) dimethyl sulfate, KOH, tetrahydrofu...
Embodiment 1 5
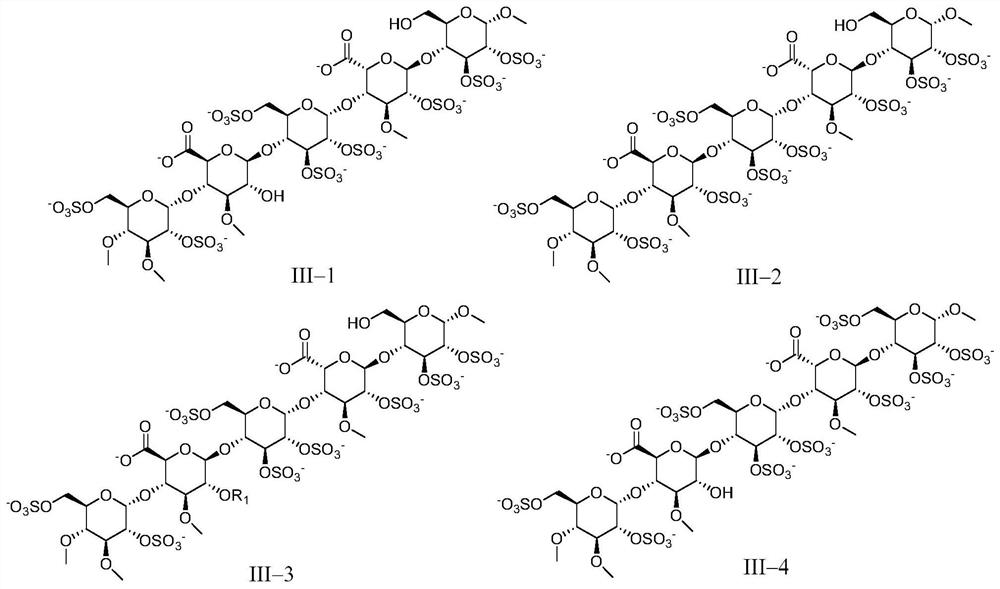
[0158] The preparation of embodiment 1 pentasaccharide III-1 (sodium salt)
[0159] synthetic route:
[0160]
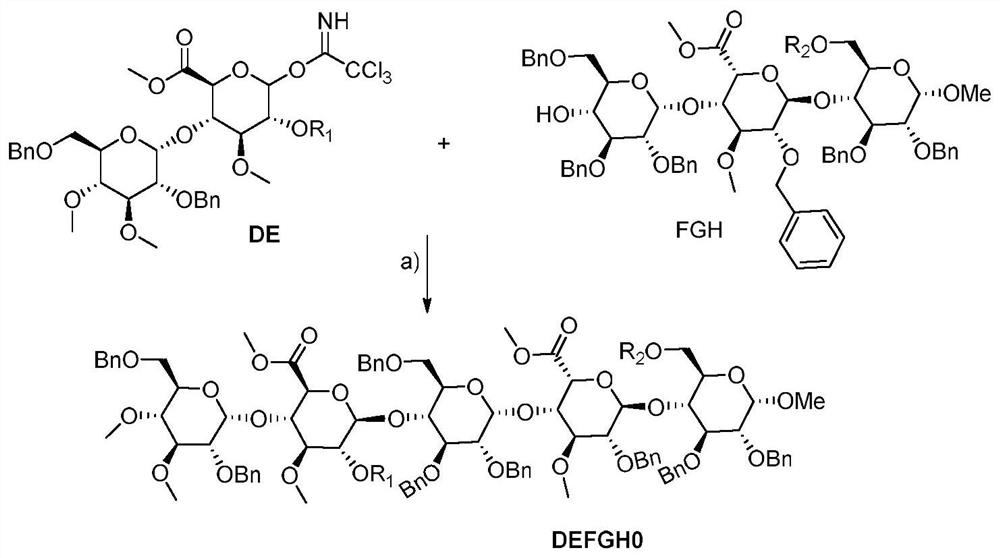
[0161] a) TMSOTf, dichloromethane, b) palladium on carbon, hydrogen, c) sulfur trioxide triethylamine salt, DMF, sodium bicarbonate, dowex-50x4, d) 4N NaOH, sephadex g25
[0162] Preparation of compound DEFGH10: Dissolve DE5 (29g) and FGH6 (45g) in anhydrous dichloromethane, add 1g of TMSOTf dropwise at 0°C, add triethylamine to quench after reacting for 1h, and obtain DEFGH10 (59g, Yield 87%). 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55(m,5H),7.34-7.29(m,40H),5.99(d,1H),5.60(m,4H),5.17(dd,J=9.0,3.4Hz,1H),4.64 –4.62(m,18H),4.49-4.19(m,5H),4.00(m,2H),3.80-3.77(m,8H),3.70(s,6H),3.61(d,2H),3.50(d ,3H),3.41-3.40(m,15H),3.36(m,2H),2.02(s,3H).MS(ESI):1843.8[M+Na] +
[0163] Preparation of compound DEFGH11: Dissolve DEFGH10 (59 g) in anhydrous methanol, add 10% palladium carbon, reduce with hydrogen under normal pressure for 24 h, filter and spin dry to obtain DEFGH11 (34....
Embodiment 2 5
[0167] The preparation of embodiment 2 pentasaccharide III-2 (sodium salt)
[0168] synthetic route:
[0169]
[0170] Referring to the method of Example 1, DE9 and FGH6 were used as raw materials to react, and then the target compound was obtained through hydrogenolysis reaction to remove benzyl group, sulfation reaction and hydrolysis reaction.
[0171] Compound DEFGH20:
[0172] 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55(m,5H),7.34-7.29(m,45H),5.60(m,5H),4.64–4.62(m,20H),4.49-4.19(m,3H),4.00(m, 2H),3.80-3.77(m,10H),3.70(s,6H),3.61(d,2H),3.50(d,3H),3.41-3.40(m,15H),3.36(m,2H),2.02 (s,3H).MS(ESI):1891.8[M+Na] +
[0173] Compound DEFGH21:
[0174] 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55(m,5H),5.40(m,5H),4.77(d,5H),4.71(d,2H),4.64–4.62(m,2H),4.49-4.19(m,5H) ,4.10(m,3H),3.94-3.80(m,8H),3.70(d,6H),3.60-3.50(m,8H),3.41-3.40(m,15H),3.30(m,2H).MS (ESI):1081.4[M+Na] +
[0175] Compound DEFGH22:
[0176] 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55(m,5H),5.54(m,5H),5.30-5.24(m,7H),,4.64–...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com