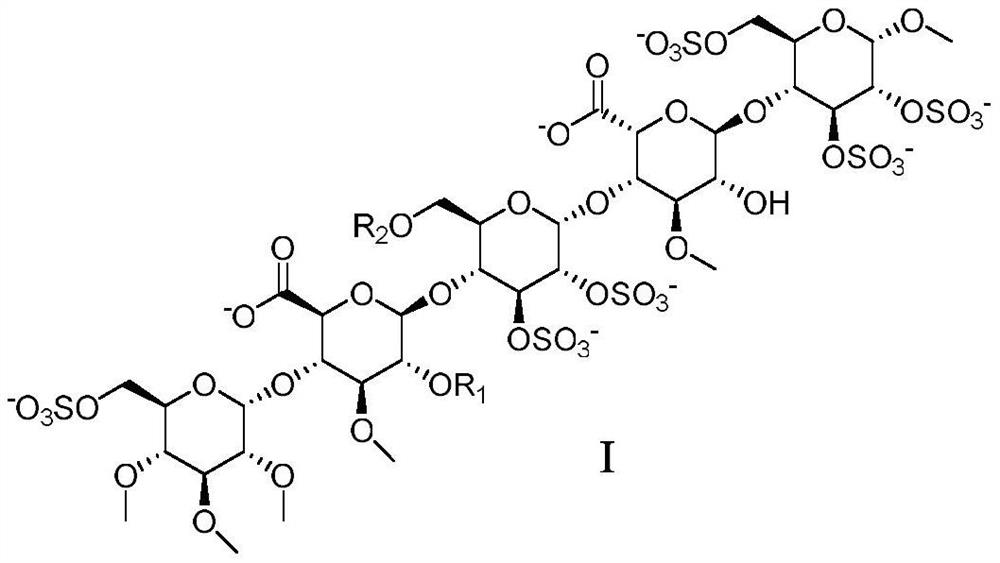
Anticoagulant pentasaccharide compound and its preparation method and medical application
A compound and composition technology, applied in the field of pentasaccharide compounds, can solve the problems of increased bleeding risk and achieve the effects of simplified preparation method, long half-life in vivo, and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] Preparation of Compounds D1-D6:
[0077] Refer to Bioorganic & Medicinal Chemistry Letters, 2009, 19(14), 3875-3879.
[0078] The second part preparation of monosaccharide E ring
[0079] synthetic route:
[0080]
[0081] a) dimethyl sulfate, KOH, tetrahydrofuran, b) trifluoroacetic acid, water, c) pyridinium p-toluenesulfonate, benzaldehyde dimethyl acetal, d) ethyl acetate, acetic anhydride, triethylamine, DMAP, e) boron trifluoride ether, p-methoxyphenol, f) sodium methoxide, methanol, g) preparation E7: potassium hydroxide, benzyl bromide, preparation E8: potassium hydroxide, dimethyl sulfate, h) acetic acid, water , i) TEMPO, BAIB, dichloromethane, water, j) iodomethane, potassium bicarbonate, acetonitrile
[0082] Preparation of Compounds E1-E4:
[0083] Reference Tetrahedron, 2012, 68(36), 7386-7399.
[0084] Preparation of Compound E5:
[0085] Dissolve E4 (366g) and 130g of p-methoxyphenol in 366ml of anhydrous dichloromethane, inject 280g of boron tr...
Embodiment 1
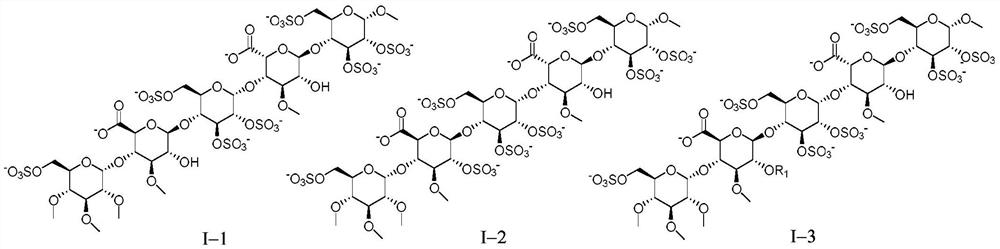
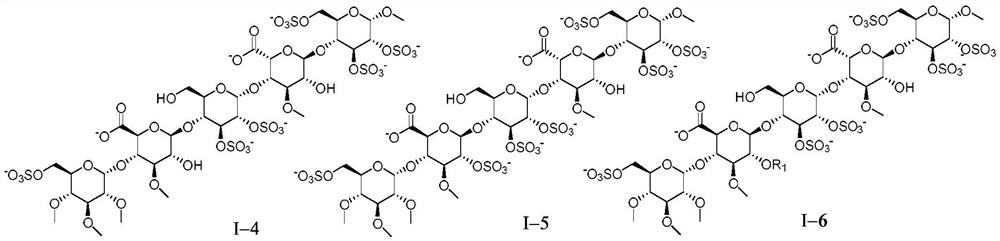
[0201] Example 1 Preparation of Pentasaccharide I-1
[0202] synthetic route:
[0203]
[0204] Preparation of compound DEFGH10:
[0205] Dissolve DE7 (29g) and FGH3 (47g) in anhydrous dichloromethane, add 1g of TMSOTf dropwise at 0°C, add triethylamine to quench after reacting for 1h, and perform column chromatography to obtain DEFGH10 (59g, yield 85%) . 1 H NMR (300MHz, CDCl 3 )δ7.34-7.29(m,35H),5.99(d,J=8Hz,,1H),5.60(m,3H),5.17(dd,J=9.0,3.4Hz,2H),4.64–4.62(m ,16H),4.23-4.19(m,4H),4.00(m,3H),3.80-3.77(m,6H),3.70(s,6H),3.61(d,3H),3.50(d,3H), 3.41(d,15H),3.36(m,3H),2.02(s,3H).MS(ESI):1705.7[M+Na] +
[0206] Preparation of Compound DEFGH11:
[0207] Dissolve DEFGH10 (59 g) in anhydrous methanol, add 10% palladium carbon, reduce under hydrogen pressure for 24 h, filter and spin dry to obtain DEFGH11 (36.9 g, yield 100%). 1 H NMR (300MHz, CDCl 3)δ5.99(d,J=3.6Hz,1H),5.60(d,1H),5.40(m,2H),5.17(dd,J=9.0,3.4Hz,2H),4.77(d,2H), 4.71(d,2H),4.64–4.62(m,2H),4.23-4.19(m,4H),3....
Embodiment 2
[0212] Example 2 Preparation of Pentasaccharide I-2
[0213] synthetic route:
[0214]
[0215] Preparation:
[0216] Referring to the method of Example 1, DE8 and FGH3 were used as raw materials to react, and then the pentasaccharide I-2 was prepared after hydrogenolysis reaction to remove benzyl group, sulfation reaction and hydrolysis reaction.
[0217] Compound DEFGH40: 1 H NMR (300MHz, CDCl 3 )δ7.34-7.29(m,35H),5.99(d,J=8Hz,,1H),5.60(m,3H),5.17(dd,J=9.0,3.4Hz,1H),4.64–4.62(m ,18H),4.23-4.19(m,3H),4.00(m,3H),3.80-3.77(m,8H),3.70(s,6H),3.61(d,3H),3.50(d,3H), 3.41(d,15H),3.36(m,3H),2.02(s,3H).MS(ESI):1705.7[M+Na] +
[0218] Compound DEFGH41: 1 H NMR (300MHz, CDCl 3 )δ5.99(d,J=3.6Hz,1H),5.60(d,1H),5.40(m,3H),5.17(dd,J=9.0,3.4Hz,1H),4.77(d,3H), 4.71(d,2H),4.64–4.62(m,2H),4.23-4.19(m,3H),4.10(m,1H),3.94(m,3H),3.90(m,4H),3.80-3.76( m,5H),3.70(d,6H),3.60-3.50(m,8H),3.41-3.40(m,18H),3.30(m,2H),2.02(s,3H).MS(ESI):1033.4 [M+Na] +
[0219] Compound DEFGH42: 1 H NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com