Synthesis method and application of dialkynyl bridged monoperylene imide dimer n-type semiconductor material
A monoperylene imide dimer, n-type semiconductor technology, applied in semiconductor devices, semiconductor/solid-state device manufacturing, electric solid-state devices, etc., can solve the problems of cumbersome synthesis process, difficult purification, harsh reaction conditions, etc. Wide application prospects, high conversion efficiency, and the effect of large electron affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
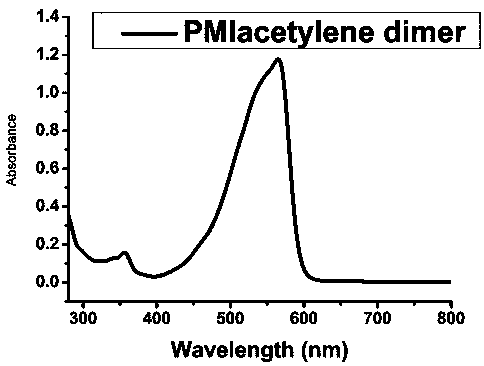
[0019] Under anaerobic conditions, use high-purity nitrogen to exhaust the air in the reaction vessel, add 3.2mmol of compound M1, 2.1mmol of cuprous iodide and 500mL of tetrahydrofuran into the reaction vessel, isolate the air and inject 50mL of anhydrous triethylamine, and then add 0.43 mmolPd (PPh 3 ) 4 Catalyst, after exhausting the oxygen, stir the mixture at 71°C for 72h, then lower it to 25°C and let it stand for 24h to turn into a red organic gel, then add 1L of deionized water and 500mL of dichloromethane in turn, shake fully and filter, filter The cake was washed 3 times with anhydrous methanol, and then the filter cake was vacuum-dried to obtain red needle-shaped crystals, which were dried in the air to obtain the small molecule semiconductor material perylene monoimide N-2-octyl-dodecyl-diacetylene Base-perylene monoimide N-2-octyl-dodecyl compound.
[0020] The ultraviolet-visible absorption spectrum figure of the small molecular semiconductor material prepared ...
Embodiment 2
[0022] Under anaerobic conditions, use high-purity nitrogen to exhaust the air in the reaction vessel, add 6.24mmol of compound M2, 4.2mmol of cuprous iodide and 700mL of tetrahydrofuran into the reaction vessel, isolate the air and inject 50mL of anhydrous triethylamine, and then add 0.61 mmolPd (PPh 3 ) 4 Catalyst, after exhausting the oxygen, stir the mixture at 71°C for 72h, then lower it to 25°C and let it stand for 24h to turn into a red organic gel, then add 1L of deionized water and 500mL of dichloromethane in turn, shake fully and filter, filter The cake was washed 3 times with anhydrous methanol, and the filter cake was vacuum-dried to obtain red needle-shaped crystals, which were dried in the air to obtain the small molecule semiconductor material perylene monoimide N-2-ethyl-hexyl-bisethynyl-perylene Monoimide N-2-ethyl-hexyl compound.
Embodiment 3
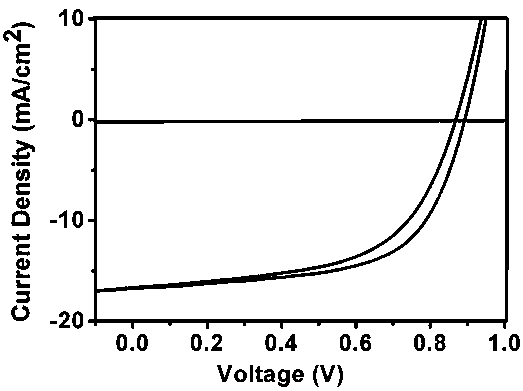
[0024] 1.5g of the small molecule semiconductor material perylene monoimide N-2-octyl-dodecyl-bisethynyl-perylene monoimide N-2-octyl-dodecyl obtained in Example 1 Mix the compound with 8.5g ITIC evenly, add 1L o-dichlorobenzene to dissolve, add 10g PBDB-T and heat to 110°C and stir for 2h, and prepare a layer thickness of 110nm thin film, and then vapor-deposit a molybdenum oxide hole transport layer with a thickness of 8nm and silver with a thickness of 100nm by vacuum evaporation to prepare a metal electrode on it, and finally obtain an organic solar cell.
[0025] In the simulated sunlight (the xenon light source purchased from Beijing Changtuo Technology Co., Ltd., without filters, and the irradiance meter purchased by Beijing Normal University Optical Instrument Factory for calibration, the light intensity is 100mW / cm 2 ), the short-circuit current of the organic solar cell is 18mA / cm 2 , the open circuit voltage is 0.88V, the fill factor is 0.60, and the energy convers...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com