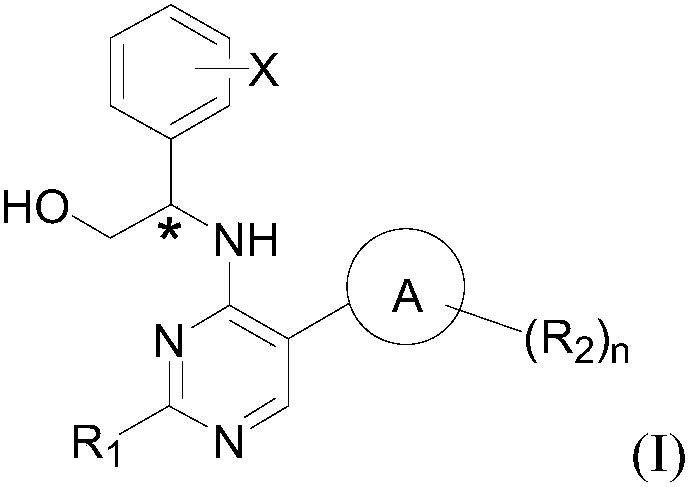
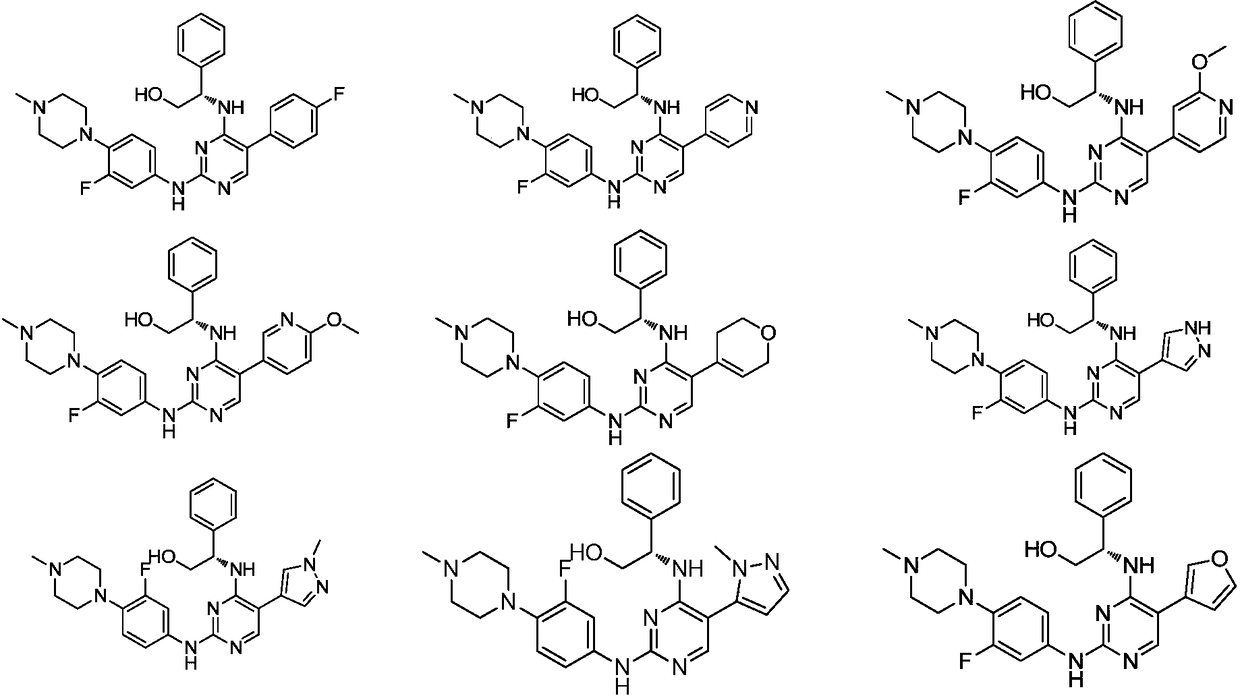
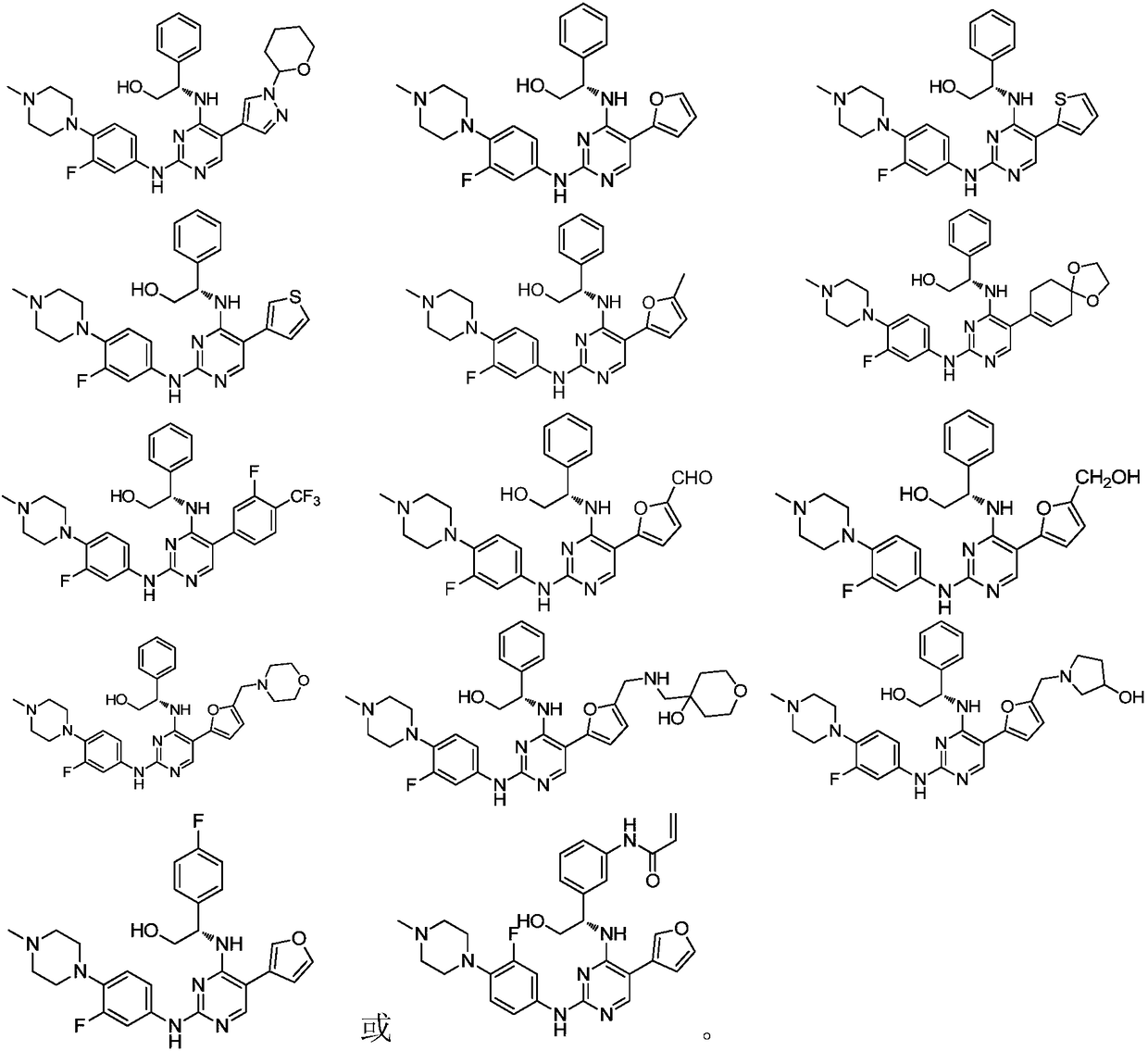
5-position ring substituted 2, 4-diaminopyrimidine compound with phenylglycinol structure, preparation and application thereof
A compound and metabolite technology, applied in the field of 2,4-diaminopyrimidine compounds, can solve the problems of lethal myelosuppressive toxicity, potential safety hazards, poor selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0113] The pharmaceutically acceptable salts of the compounds of the present invention can be prepared by the direct salt-forming reaction between the free base of the compound and an inorganic or organic acid. The inorganic or organic acid may be selected from hydrochloric acid, sulfuric acid, phosphoric acid, nitric acid, hydrofluoric acid, hydrobromic acid, formic acid, acetic acid, picric acid, citric acid, maleic acid, methanesulfonic acid, trifluoromethanesulfonic acid, ethanesulfonic acid acid and p-toluenesulfonic acid etc.
[0114] Since the compound of the present invention has excellent inhibitory activity against FLT3 kinase (Kinase) and mutant FLT3-ITD, the compound of the present invention and its various crystal forms, pharmaceutically acceptable inorganic or organic salts, hydrates or solvates, And the pharmaceutical composition containing the compound of the present invention as the main active ingredient can be used to treat, prevent and relieve diseases rela...
Embodiment 1
[0131]
[0132] Synthesis of Compound 1-3:
[0133] Weigh compound 1-1 (4.5g, 20mmol), compound 1-2 (3.3g, 24mmol) in a single-necked bottle, add absolute ethanol, and then add N,N-diisopropylethylamine (6.6ml , 40mmol), stirred at room temperature for 6h. After the reaction, a large amount of white solids precipitated, and were filtered by suction to obtain compound 1-3, 5.48 g. The mother liquor obtained by suction filtration was extracted with ethyl acetate and water, and the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. Separation by column chromatography gave Compound 1-3, 1.04g.
[0134] Synthesis of compounds 1-4:
[0135] Weigh compound 1-3 (78mg, 0.24mmol), compound 3-fluoro-4-(4-methyl-1-piperazinyl) aniline (42mg, 0.2mmol) in a one-mouth bottle, add 3ml of isopropanol, Then D(+)-10-camphorsulfonic acid (93mg, 0.4mmol) was added, and the mixture was refluxed at 85°C overnight. After...
Embodiment 17
[0145]
[0146] Weigh compound 1-4 (100mg, 0.2mmol), compound 17-1 (36mg, 0.26mmol) in a single-necked bottle, add Pd(OAc) 2 (26mg, 0.12mmol), S-Phos (98mg, 0.24mmol), potassium phosphate (106mg, 0.50mmol). Then add 6ml tetrahydrofuran and 1ml water as solvent, nitrogen protection, react at 90°C for 4h. After the reaction, extracted with ethyl acetate and water, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by column chromatography to obtain compound S17, 54mg, compound S17 as a yellow solid. analyze data: 1 H NMR (400MHz, DMSO-d 6 )δ9.61(s,1H),9.57(s,1H),8.51(s,1H),7.71(d,J=3.8Hz,1H),7.57(d,J=15.8Hz,1H),7.46( d, J=7.4Hz, 3H), 7.35(t, J=7.6Hz, 2H), 7.25(d, J=7.2Hz, 2H), 7.13(d, J=3.8Hz, 1H), 6.91(t, J=9.4Hz, 1H), 5.32(s, 1H), 5.19(t, J=5.2Hz, 1H), 3.78(ddt, J=40.0, 11.3, 5.5Hz, 2H), 2.97(s, 4H), 2.50(d,J=5.2Hz,4H),2.24(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com