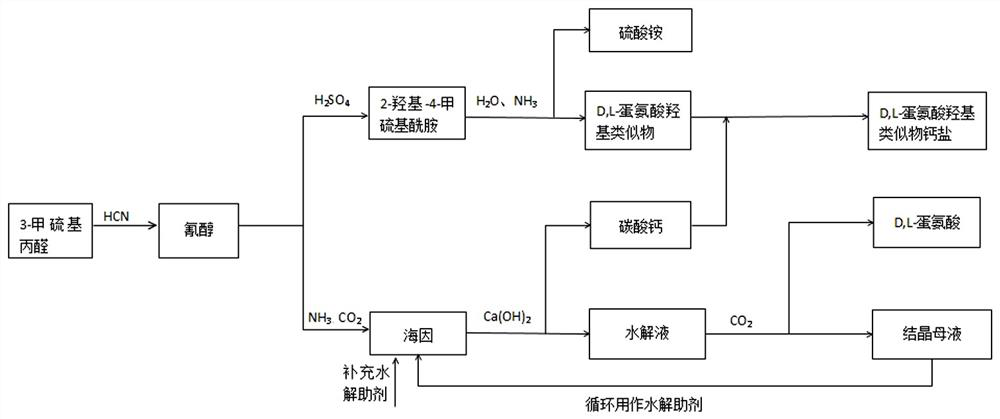
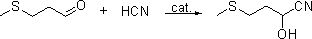A clean process for the co-production of d,l-methionine, d,l-methionine hydroxyl analogs and their calcium salts
A technology of methionine hydroxyl group and analogs, applied in the chemical industry, can solve the problems of low extraction rate, consumption of alkali, and high extraction cost, and achieve the effects of increasing extraction rate, reducing circulation volume and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 cyanohydrin
[0059] 6mol content of 99.5% 3-methylthiopropanal is transferred to the tank or tower reactors A and B connected in series, each adding 10~50wt% industrial desalted water relative to 3-methylthiopropanal After the citric acid-sodium hydroxide buffer salt catalyst of 0.5~5wt%, pass into the Angle method hydrogen cyanide gas (HCN content 7 ~10wt%), the tail gas after the reaction enters the secondary reactor or tower B (reaction temperature 0~25°C) for further absorption reaction, the tail gas of the secondary reactor enters the tail gas treatment system, and the reaction is kept for 1~12h, and analyzed by liquid chromatography. The primary reactor obtains cyanohydrin with a conversion rate of over 99%, and the secondary reaction obtains a mixture of cyanohydrin and 3-methylthiopropionaldehyde with a conversion rate of 20-60%.
[0060] The cyanohydrin product in the primary reactor A is transferred to obtain an aqueous cyanoh...
Embodiment 2
[0061] The preparation of embodiment 2 liquid methionine
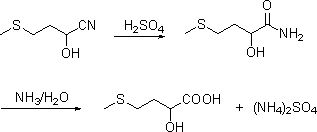
[0062] Transfer one batch of 410g cyanohydrin (mass fraction 76wt%) obtained in Example 1 into a 316 reactor, add 0.5~1 molar equivalent of cyanohydrin concentrated sulfuric acid (98wt%), and keep warm at 30~70°C for 1~5h to generate The hydration reaction of cyanohydrin, chromatographic analysis of cyanohydrin conversion is complete, add desalinated water, carry out hydrolysis reaction at 90~130°C, heat preservation reaction for 1~5h, chromatographic analysis reaction conversion is complete, add 0.5~1.2 cyanohydrin molar equivalent 30wt% ammonia water and stir Neutralize for 10-30 minutes, let stand to separate layers, extract the water phase, further concentrate the oil phase under reduced pressure and separate the ammonium sulfate precipitated therein, and finally obtain the concentrated liquid methionine solution, add water to 88-95wt%.
Embodiment 3
[0063] The preparation of embodiment 3 solid methionine
[0064] One batch of 390g cyanohydrin (mass fraction 70wt%) obtained in Example 1 was transferred to a zirconium material autoclave, and 1~1.2 cyanohydrin molar equivalents of carbon dioxide and 2~2.2 cyanohydrin molar equivalents of 20~30wt% ammonia water were added at 140 Stir at ~160°C and 0.5~1.5MPa (speed 100~200r / min) and react for 10~60min to prepare hydantoin solution. Mix the obtained hydantoin solution with 0.95~1.2 hydantoin molar equivalents of potassium ions (in the form of potassium carbonate) and 0.8~1.2 hydantoin molar equivalents of calcium hydroxide, transfer to a zirconium material autoclave, and heat at 170~200 ° C, 0.8 Stirring under ~2.0MPa (rotation speed 110~300r / min) reaction 10~60min hydrolysis to prepare hydrolyzate and calcium carbonate precipitate, suction filter to obtain calcium carbonate precipitate and hydrolyzate, hydrolyzate after stripping, bubbling, vacuum distillation, etc. Remove t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com