Synthesizing method of sulfentrazone
A synthetic method, the technology of sulfentranacil, which is applied in the field of synthesis of sulfentrazone, can solve the problems affecting product quality and yield, strong corrosion of methanesulfonyl chloride, low yield of sulfentrazone, etc., and achieve the product The effect of good quality, high product yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
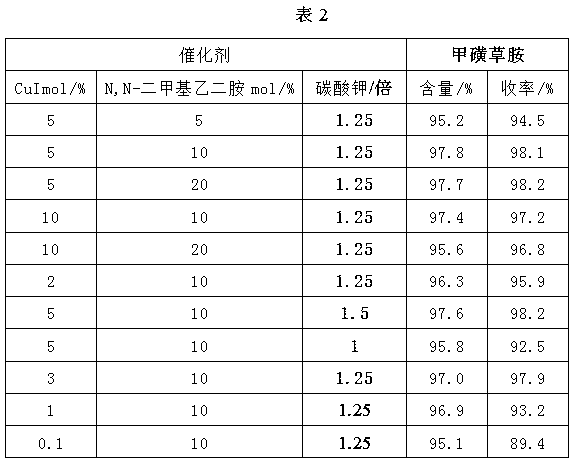
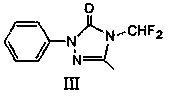
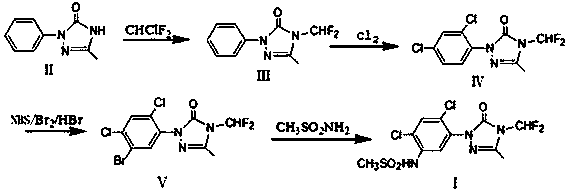
[0031]Dissolve 17.6g of compound (II) in 88g of DMF, add 20.7g of potassium carbonate, raise the temperature to 140°C, and feed 10.5g of difluorochloromethane. After the reaction, cool down to room temperature and filter out the potassium salt; filter the filtrate at 20 Chlorine gas flow at ℃, and stop the chlorine gas flow when the central control is not turned to less than 1%. After the reaction, DMF is removed to obtain the intermediate (Ⅲ); 50% acetic acid aqueous solution is added to the intermediate (Ⅲ), and the temperature is raised to 80°C to start the flow. Chlorine gas, stop the chlorine gas when the central control is not turned to less than 1%, cool down, crystallize, filter and dry to obtain 25.6g of intermediate (Ⅳ), the content of which is 98.4% by GC.
[0032] Weigh 10g of intermediate (Ⅳ) and dissolve in acetic acid, add 0.05g of iodine, raise the temperature to 30°C, start to add 3.4g of bromine dropwise, control the dropping time between 2-3h, after the end, ...
Embodiment 2
[0035] Prepare sulfentrazone according to the method of Example 1, the difference is: the preparation process of the intermediate (Ⅴ) is: weigh 10g of the intermediate (Ⅳ) and dissolve it in 96% sulfuric acid, add 0.05g of iodine, and heat up to 30°C , start to add 3.4g of bromine dropwise, and control the dropping time between 2-3h. After the end, add 2.4g of hydrogen peroxide dropwise for 2-3h, keep warm for 1h after adding, add 30g of water, cool down, filter, wash, and dry , 12.5 g of intermediate (Ⅴ) was obtained, the content detected by HPLC was 97.8%, and the yield based on intermediate (Ⅳ) was about 98.1%.
Embodiment 3
[0037] Prepare sulfentrazone according to the method of Example 1, the difference is: the preparation process of the intermediate (Ⅴ) is: weigh 10g of the intermediate (Ⅳ) and dissolve it in 96% sulfuric acid, add 0.05g of iodine, and heat up to 60°C , start to add 3.4g of bromine dropwise, and control the dropping time between 2-3h. After the end, add 2.4g of hydrogen peroxide dropwise for 2-3h, keep warm for 1h after adding, add 30g of water, cool down, filter, wash, and dry 12.4 g of intermediate (Ⅴ) was obtained, the content detected by HPLC was 97.4%, and the yield based on intermediate (Ⅳ) was about 96.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com