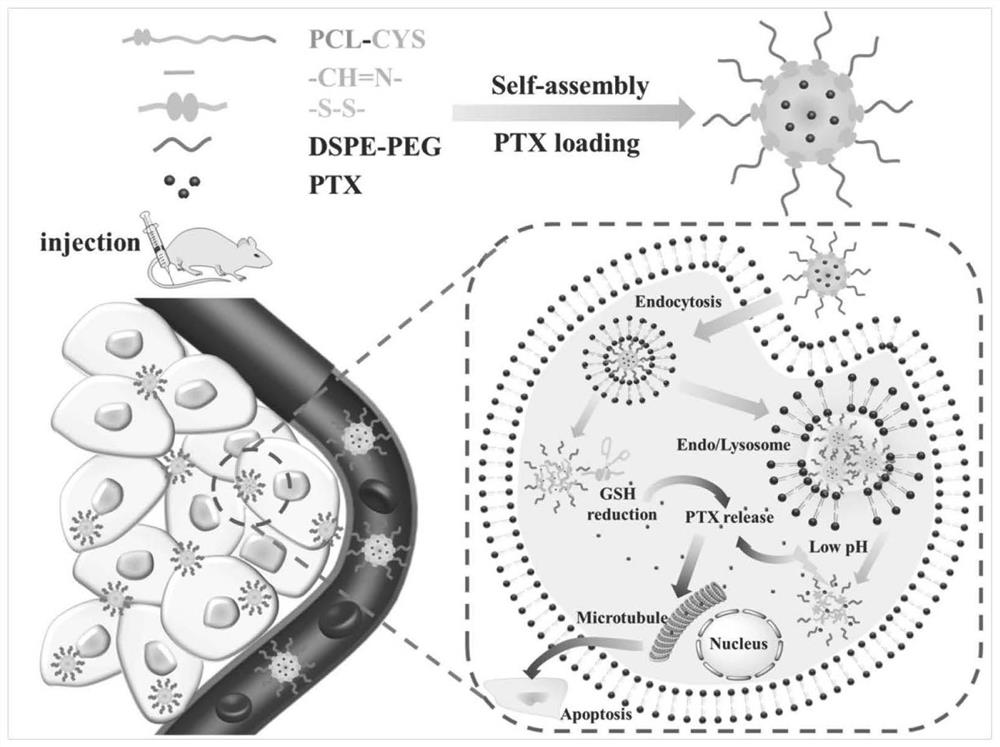
Ph/reduction double-sensitive carrier material formed by cys and its derivatives and polyester polymer and its preparation method and application
A polymer and sensitive technology, which is applied in the field of functional polymer materials and biomedicine, can solve the problems of poor biological safety, poor circulation stability, poor targeting, etc., and achieve short reaction cycle, excellent biological safety and few reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
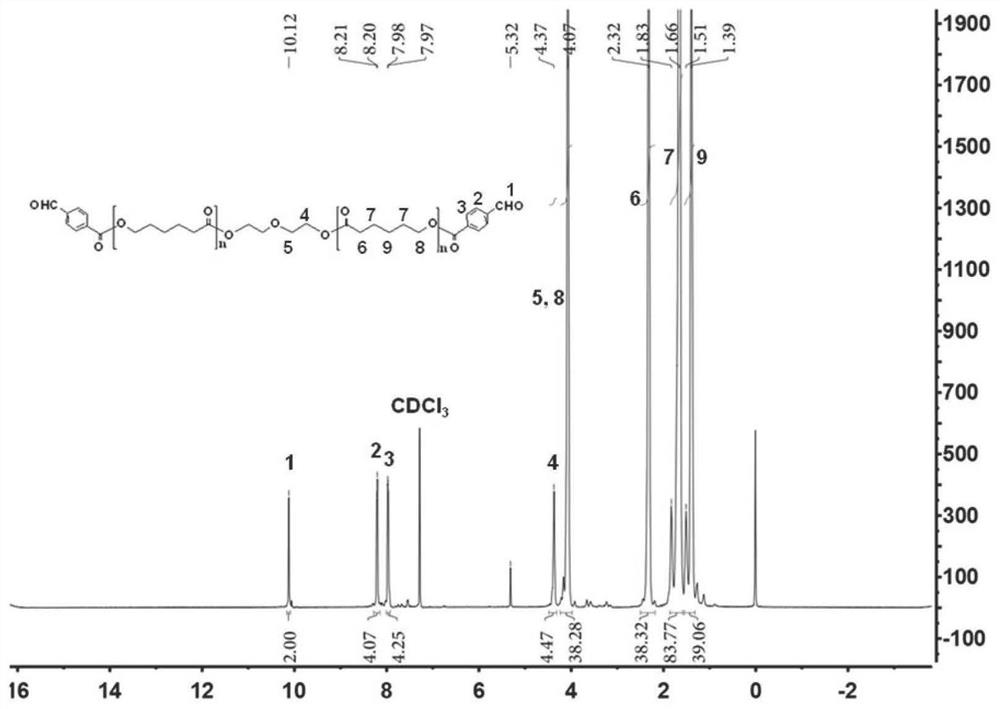
[0112] Synthesis of the polyester polymer PCL-CHO of embodiment 1 formylation
[0113] 1, the synthetic of the polyester polymer PCL-CHO of aldehydization, comprises the following steps:
[0114] (1) Polycaprolactone (PCL 2K ) (4.03g, 2.01mmol), 4-chlorobenzoic acid (p-CBA) (0.82g, 5.46mmol) and 4-dimethylaminopyridine (DMAP) (0.33g, 0.27mmol) were blended in a 50mL round bottom In the flask, after adding 30mL of anhydrous dichloromethane, place it in a cold trap, control the temperature at 0°C, add dicyclohexylcarbodiimide (DCC) (1.34g, 6.51mmol) under nitrogen protection environment, and set the temperature at 0°C Stir for 24h to obtain an aldylated polyester product;
[0115] (2) after the obtained product is rotary evaporated, pass through silica gel column chromatography, first use the dichloromethane / acetone of volume ratio 5:1 as eluent elution, remove by-product DCU; / methanol was used as the eluent for purification, and the product was further purified to finally o...
Embodiment 2
[0121] Embodiment 2 has the synthesis of pH / reduction double sensitive polymer Cys-PCL
[0122] 1. The synthesis of the polymer Cys-PCL with pH / reduction double sensitivity comprises the following steps:
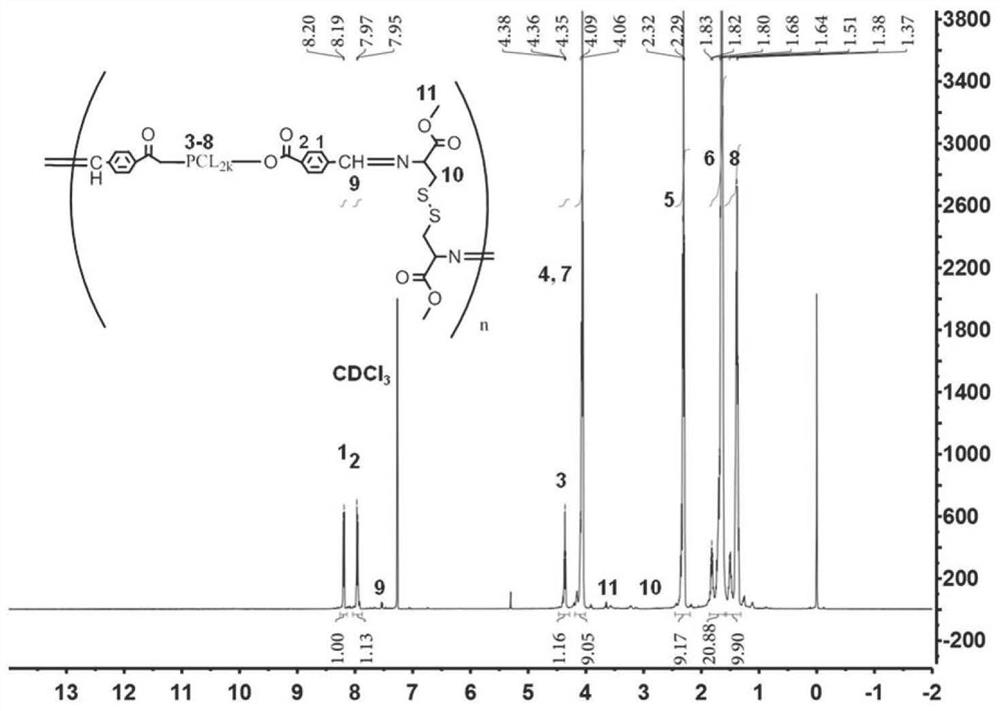
[0123] (1) Put L-cystine dimethyl ester dihydrochloride (Cys) (0.34g, 1.01mmol) and aldylated polyester polymer PCL-CHO (2.05g, 1.02mmol) in a 50mL circle In the bottom flask, add 30mL ethanol, reflux overnight, and after rotary evaporation, the resulting product is purified by Sephadex LH-20 gel chromatography with methanol as the eluent, and finally a yellow waxy solid is obtained, which is pH / reduction The double-sensitive polymer Cys-PCL has a reaction yield of 86.7%.
[0124] The structural formula of gained polymer Cys-PCL is as shown in formula (I):
[0125]
[0126] 2. Results
[0127] The H NMR spectrum of the polymer Cys-PCL is as image 3 shown. Depend on image 3 It can be seen that the signal at ~10.12ppm corresponds to the disappearance of the proton a...
Embodiment 3
[0129] Example 3 Preparation, Characterization, In Vitro Drug Release and Cell-to-Pair Experiment of Cys-PCL@PTX Drug-loaded Nanoparticles
[0130] 1. Prepare Cys-PCL@PTX drug-loaded nanoparticles by the following nanoprecipitation method, including the following steps:
[0131] (1) 10mg Cys-PCL, 2.5mg DSPE-PEG 2K , 2mg of PTX were dissolved in 1.0mL DMSO together as the oil phase, and under stirring, the oil phase was added dropwise to 20mL of PBS solution at a rate of 0.05mL / sec to obtain a drug-loaded nanoparticle solution with uniform and stable particle size;
[0132] (2) Concentrate the obtained nanoparticle solution by ultrafiltration at 2500 rpm, dilute to 2 mL, and freeze-dry to obtain Cys-PCL@PTX drug-loaded nanoparticles.
[0133] 2. Characterize the obtained Cys-PCL@PTX drug-loaded nanoparticles, and the results of in vitro drug release and cytotoxicity are as follows:
[0134] (1) As detected by DLS, the particle size of the drug-loaded nanoparticles of the pres...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com