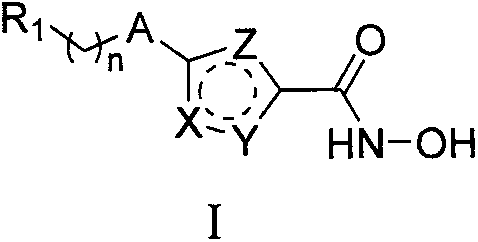
Hydroxamic acid-containing substituted heterocyclic compound as well as preparation method and application thereof
A technology of hydroxamic acid and compound, applied in the field of medicinal chemistry, can solve the problems of poor selectivity, inability to apply, poor stability of phosphatase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
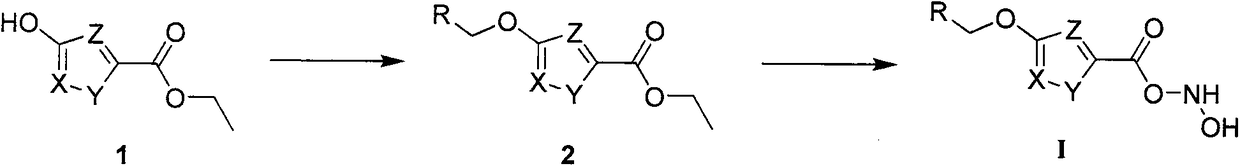
[0029] Example 1 Synthesis of some compounds of the present invention.
[0030] Preparation of 3-(decyloxy)-N-hydroxyisoxazole-5-carboxamide:
[0031]
[0032] Dissolve 1 g (6.99 mmol) of 3-hydroxyisoxazole-5-methyl carboxylate in 20 mL of acetone, add 1.93 g (13.98 mmol) of potassium carbonate, 50 mg of potassium iodide, and 1.85 g (8.39 mmol) of bromodecane, and heat up to The reaction was refluxed for 10h and cooled to room temperature. The reaction solution was filtered, the filter cake was washed three times with dichloromethane, the organic phases were combined, the solvent was spin-dried, and purified by column chromatography (petroleum ether: ethyl acetate = 8:1) to obtain 1.88 g of a white solid, with a yield of 95%. Dissolve the obtained product in 10 mL of methanol, add 20 mL of 1.76M methanol solution of potassium hydroxylamine, stir at room temperature for 6 h under nitrogen protection, add 15% HCl aqueous solution to make it acidic, and precipitate the produc...
Embodiment 2
[0048] Example 2 Experiment of compounds inhibiting acid sphingomyelinase activity.
[0049] Acid sphingomyelinase can hydrolyze sphingomyelin in cells to generate ceramide. For a certain amount of fluorescently labeled reaction substrates, different enzyme activities catalyze and generate different amounts of products. By detecting the content of products, the level of enzyme activity can be investigated. The present invention carries out experimental design according to this principle. Extract the protein in the cultured cells, add buffer, fluorescently labeled reaction substrate, and then add different concentrations of compounds respectively, set up a blank control group, perform fluorescence analysis after the reaction, and finally calculate the IC of the compound 50 value.
[0050] The specific results are shown in the table:
[0051] Table 1: Acid sphingomyelinase inhibitory activity of some compounds of the present invention
[0052]
Embodiment 3
[0053] Example 3 Anti-atherosclerotic activity of compounds
[0054] Experimental method: 40 male ApoE knockout mice were selected and randomly divided into blank group, atorvastatin control group, I-1 high-dose administration group, and I-1 low-dose administration group. Atorvastatin is used at a dose of 12mg / Kg. The low dose of I-1 is 12mg / Kg, and the high dose is 24mg / kg. Ten mice in each group were fed with high-fat diet for 12 weeks. At the same time as high-fat feeding, intragastric administration was started. After 12 weeks of administration, the animals were sacrificed, and the aorta was cut for Oil Red O staining.
[0055] Table 2: Compound I.1 reduces aortic fatty plaque in atherosclerotic mice
[0056] group
[0057] The experimental results show that compound I-1 can effectively reduce the formation of atherosclerotic plaques in the aorta of mice fed a high-fat diet, and inhibit the occurrence of atherosclerosis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com