Preparation method of NiCo2S4-coated porous carbon skeleton for positive electrode material of lithium-sulphur battery
A cathode material, lithium-sulfur battery technology, used in battery electrodes, lithium storage batteries, non-aqueous electrolyte storage batteries, etc. Diffusion, the effect of improving cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
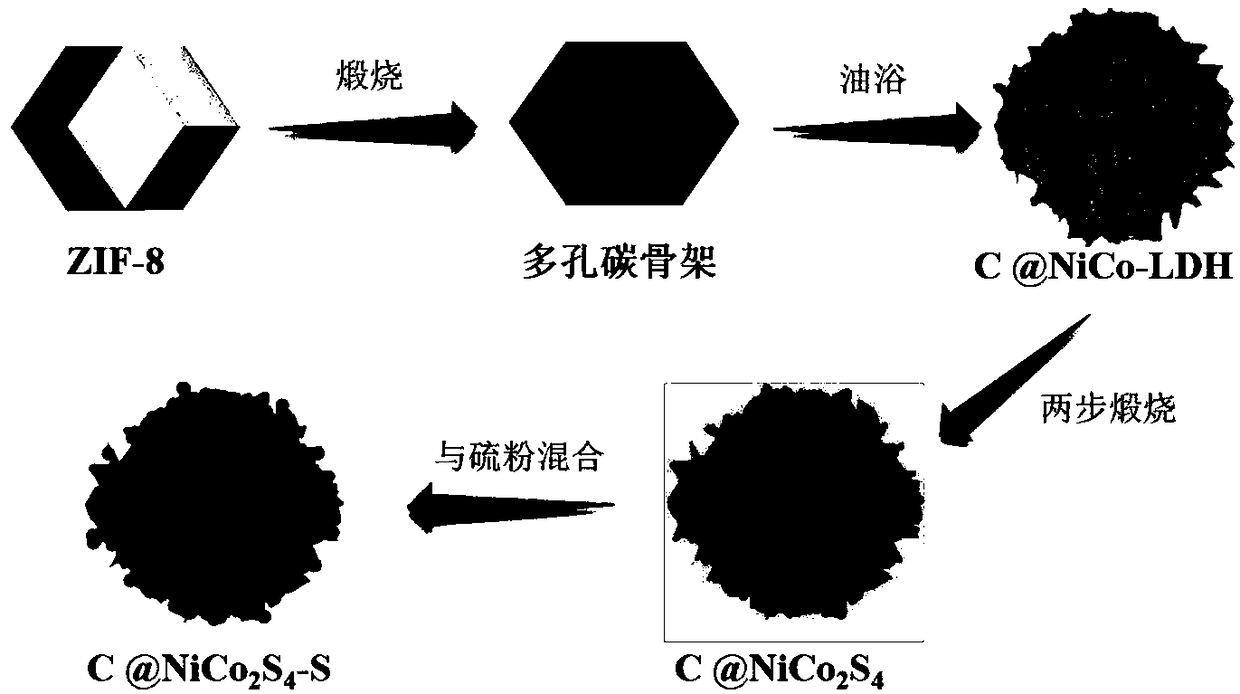
[0025] (1) Preparation of nitrogen-doped porous carbon. Weigh 7g of zinc acetate hexahydrate and dissolve it in 200ml of deionized water. Weigh 28g of dimethylimidazole and dissolve it in 200ml of deionized water. After stirring evenly, the two solutions were mixed together. After stirring for 15min, let stand for 24h at room temperature. , centrifuged 3 times with deionized water and methanol, and dried in vacuum at 60 °C for 12 h to obtain ZIF-8 powder. The ZIF-8 powder was placed in a tube furnace, calcined at 800 °C in an argon atmosphere, kept for 2 h, and the heating rate was 5 °C / min. After calcination, the furnace lid was opened, and the sample was cooled to room temperature in an argon atmosphere. , then the sample was taken out from the tube furnace, acidified with 65% concentrated nitric acid, washed with dilute hydrochloric acid to remove zinc, filtered with deionized water and alcohol, and dried at 80 °C for 12 h to obtain nitrogen Doped Porous Carbon.
[0026] ...
Embodiment 2
[0032] The difference from Example 1 is: (2) Preparation of C@NiCo-LDH composite material. The prepared porous carbon, cobalt nitrate hexahydrate, nickel nitrate hexahydrate, and urotropine were added into 40ml deionized water at a molar ratio of 10:60:30:30, stirred at room temperature for 10min, then poured into an Erlenmeyer flask, An oil bath was carried out at 90°C, condensed and refluxed for 3 hours, the reaction product was collected by centrifugation with water and alcohol, and the NiCo-LDH coated porous carbon composite material was obtained after drying at 80°C. The rest are the same as in Embodiment 1, and will not be repeated here.
[0033] Compared with the obtained material in Example 1, the growth of flaky NiCo-LDH in C@NiCo-LDH is not dense enough, resulting in the subsequent growth of flaky NiCo 2 S 4 less and unevenly distributed.
Embodiment 3
[0035] The difference from Example 1 is: (2) Preparation of C@NiCo-LDH composite material. The prepared porous carbon, cobalt nitrate hexahydrate, nickel nitrate hexahydrate, urotropine, and sodium citrate were added to 40ml of deionized water in a molar ratio of 10:120:60:60:10, and stirred at room temperature for 10 minutes. Pour it into a Erlenmeyer flask, place it in an oil bath at 90°C, condense and reflux for 6 hours, collect the reaction product by centrifugation with water and alcohol, and dry it at 80°C to obtain a NiCo-LDH-coated porous carbon composite material. The rest are the same as in Embodiment 1, and will not be repeated here.
[0036] Compared with the obtained material in Example 1, the C@NiCo-LDH composite material can grow a sheet-like NiCo-LDH structure, but it is stuck together and the specific surface area is reduced, which will also lead to a decrease in the loading of active material sulfur.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com