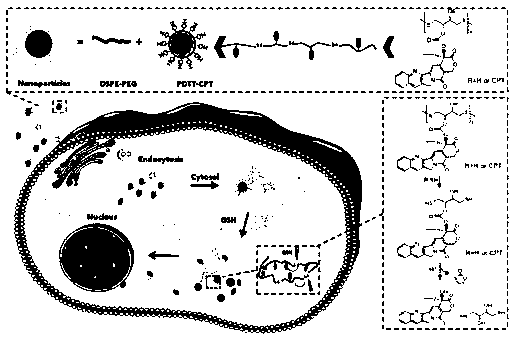
Polydithiothreitol nano system for antitumor drug delivery and preparation method and application of polydithiothreitol nano system for antitumor drug delivery
A technology of polydithiothreitol and dithiothreitol, which is applied in the field of polydithiothreitol nanosystems for anti-tumor drug delivery and its preparation, and can solve the problem of low water solubility of camptothecin and difficulty in preparing preparations, etc. problems, to achieve the effect of improving targeted delivery efficiency, stable properties, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105]The synthesis of embodiment 1 polydithiothreitol
[0106] 1. Prepare polymer polydithiothreitol (PDTT) as drug carrier by the following method, comprising the following steps:
[0107] (1) Weigh 4.62g of dithiothreitol, dissolve it with 11mL of dried dimethyl sulfoxide, and keep a high vacuum inside the reaction vessel by freezing with liquid nitrogen;
[0108] (2) After returning to room temperature, raise the temperature to 90°C while keeping stirring, and after 10 hours of reaction, remove residual dimethyl sulfoxide and product water;
[0109] (3) Add ethyl acetate, heat to dissolve the solid crude product, and then cool and precipitate the solid at room temperature. After repeated repetitions, a white solid is obtained, which is polydithiothreitol.
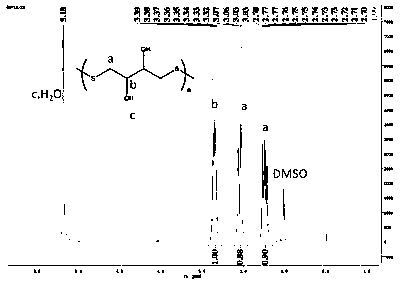
[0110] 2. Results
[0111] The NMR spectrum of polymer PDTT is as follows figure 2 shown by figure 2 It can be seen that the signal at ~5.17ppm is the absorption peak of solvent heavy water, the signal at ~3.34ppm...
Embodiment 2
[0113] The synthesis of embodiment 2 polydithiothreitol
[0114] 1. Prepare polymer polydithiothreitol (PDTT) as drug carrier by the following method, comprising the following steps:
[0115] (1) Weigh 2.31g of dithiothreitol, dissolve it with 5.5mL of dried dimethyl sulfoxide, and keep a high vacuum inside the reaction vessel by freezing with liquid nitrogen;
[0116] (2) After returning to room temperature, raise the temperature to 100°C while keeping stirring, and after 20 hours of reaction, remove residual dimethyl sulfoxide and product water;
[0117] (3) Add ethyl acetate, heat to dissolve the solid crude product, and then cool and precipitate the solid at room temperature. After repeated repetitions, a white solid is obtained, which is polydithiothreitol.
[0118] 2. Results
[0119] The prepared polydithiothreitol has a stable structure, good biocompatibility, and can carry hydrophobic antitumor drugs, and a large number of disulfide bonds in the polymer make the sys...
Embodiment 3
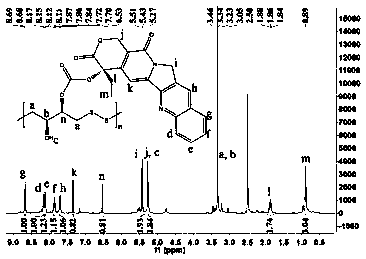
[0120] The preparation of embodiment 3 graft medicine (polydithiothreitol grafted camptothecin)
[0121] 1. Prepare the graft drug by the following method, comprising the following steps:
[0122] (1) Take a clean and dry 250mL round bottom flask, weigh 2g of camptothecin, 2.11g of 4-dimethylaminopyridine (DMAP) and dissolve in 100mL of dichloromethane, and place it on a magnetic stirrer under the protection of argon. Stir for 10 minutes;
[0123] (2) Weigh phosgene 0.57g, quickly join in the round-bottomed flask, and the system continues to stir for 30min to carry out the acylation reaction;
[0124] (3) 0.9 g of polydithiothreitol was dissolved in 30 mL of tetrahydrofuran, placed in a constant pressure funnel, dropped into a round bottom flask under the protection of argon, and grafted at room temperature for 24 hours;
[0125] (4) Concentrate the solvent to 30mL by rotary evaporation, then drop into ethyl acetate, put it in a refrigerator at 20°C overnight, filter, rinse ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com