Chitosan modified triamcinolone acetonide acetate lipidosome and preparation method thereof
A technology of triamcinolone acetonide and liposome, which is applied in liposome delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem that eye drops cannot be applied clinically and are prone to flocculation , Poor adsorption on the corneal surface, etc., to improve drug bioavailability, good therapeutic effect, and improve surface electrical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
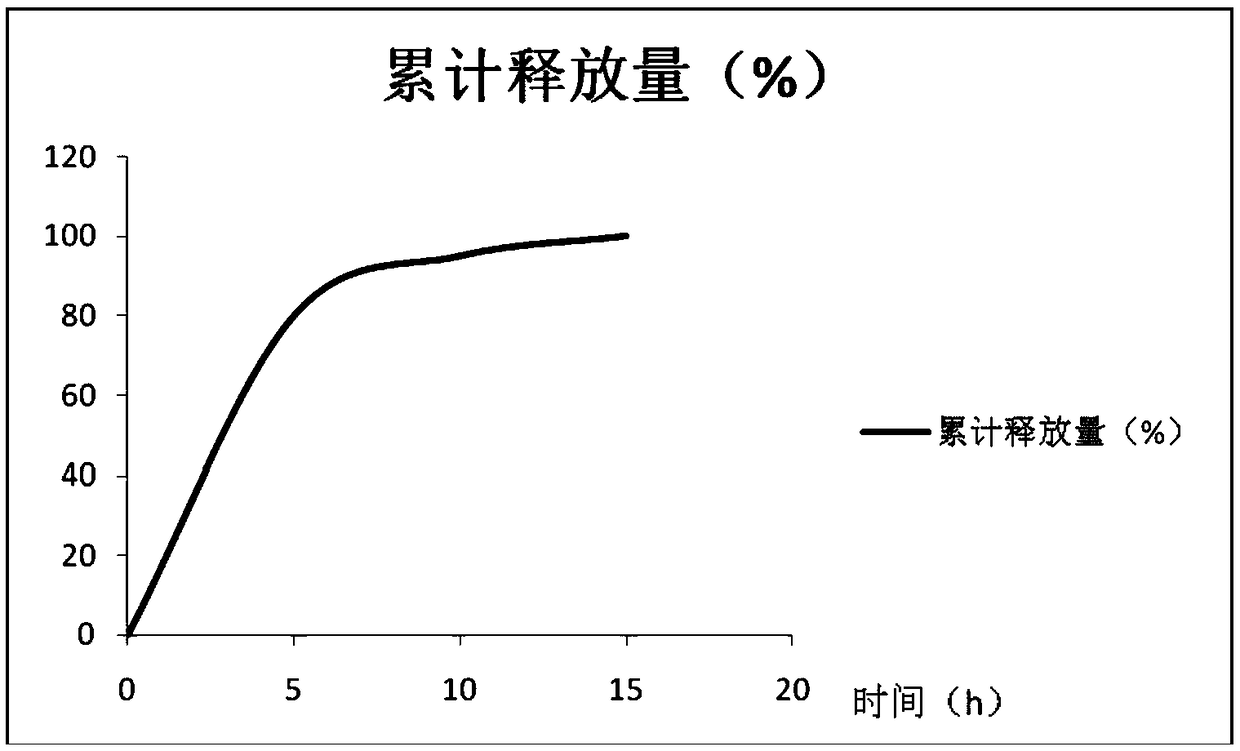
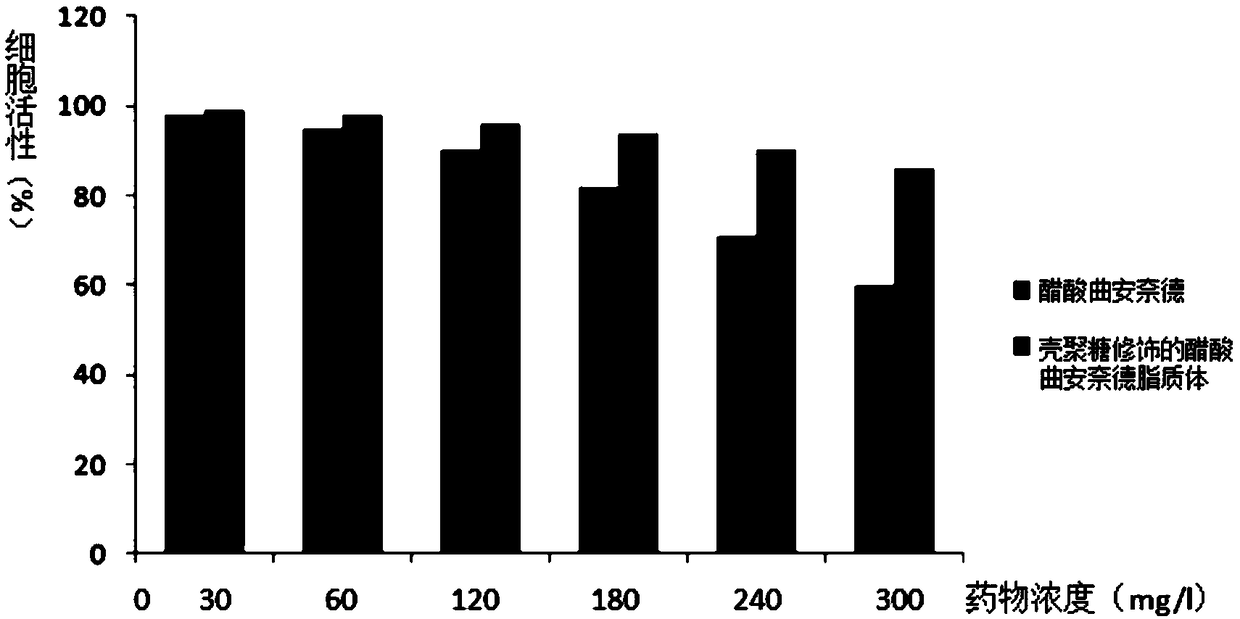
Image
Examples
Embodiment 1
[0045] Triamcinolone acetonide liposomes modified by embodiment 1 chitosan preparation
[0046] Dissolve soybean lecithin and cholesterol in chloroform at a molar ratio of 8:1, and use a rotary evaporator at 35°C at a speed of 200 rpm for 15 minutes to form a film. Place in a vacuum environment overnight to completely remove residual organic solvents.
[0047] Calcium acetate solution (120 mM) was added, and ultrasonic hydration was carried out at 45° C. for 3 h under normal pressure to obtain a blank liposome suspension with blue opalescence.
[0048] The suspension was repeatedly frozen and thawed, and filtered with a 0.22 micron microporous filter under the condition of nitrogen pressure ≤ 200 psi. This step was repeated 10 times to obtain a transparent liposome suspension.
[0049] Put the suspension into a dialysis bag, use 0.9% sodium chloride solution as the dialysate, incubate overnight at room temperature, and change the dialysate every 2 hours to completely remove f...
Embodiment 2
[0053] The preparation of the triamcinolone acetonide acetate liposome eye drops of embodiment 2 chitosan modification
[0054] The triamcinolone acetonide acetate liposome suspension wrapped in chitosan in Example 1 was mixed with sodium hyaluronate eye drops, the drug concentration was adjusted, and the concentration of triamcinolone acetonide acetate was controlled to be 1.5mg / mL to obtain chitosan Sugar-modified triamcinolone acetonide liposomal eye drops. Store at 4°C.
Embodiment 3
[0055] Embodiment 3 drug loading rate and encapsulation efficiency determination
[0056] Chromatographic conditions: the chromatographic column is a Hypersil ODS column (250mm×4.6mm, 5μm), methanol-water-ether (volume ratio 68:32:4) is the mobile phase, the ultraviolet detection wavelength is 240nm, the column temperature is 30°C, and the flow rate is 1.0mL. min -1 , the injection volume was 20 μL, and the internal standard method was used for quantification.
[0057] Draw 1ml of chitosan-modified triamcinolone acetonide liposome eye drops into a 10mL volumetric flask, add methanol to disperse and dissolve completely, filter through a 0.22 μm microporous membrane, transfer to a 10mL volumetric flask and use mobile phase to make up the volume To the scale, shake well to get the sample solution, inject 20 μL for chromatographic analysis, and determine the total mass of triamcinolone acetonide acetate. 药 .
[0058] Another equivalent amount of chitosan-modified triamcinolone ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com