Co-B/NGO composite nanometer material as well as preparation method and application thereof
A composite nanomaterial, co-b technology, applied in the field of catalytic chemistry, can solve the problems of catalyst material microstructure damage, reduce catalyst catalytic performance, low commercial application value, etc., achieve excellent catalytic performance, excellent cycle performance, and protect the microscopic shape. appearance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation method of Co-B / NGO composite nanomaterial, concrete operation steps are as follows:
[0033] Step 1) Precursor preparation, weigh 1mmol of nitrogen-doped graphene and 6mmol of cobalt nitrate hexahydrate, add to deionized water for ultrasonic dispersion, then add sodium borohydride at a ratio of 1:10 for in-situ reduction, and then The solution obtained above is centrifuged, and the precursor is obtained after washing;
[0034] Step 2) Preparation of Co-B / NGO composite nanomaterials. Put the precursor in a refrigerator, freeze for 24 hours, and freeze-dry at -48°C to obtain Co-B / NGO composite nanomaterials.
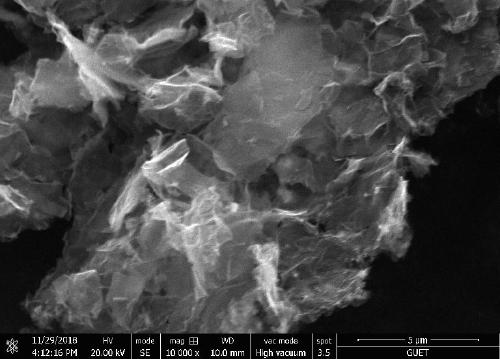
[0035] Co-B / NGO composite nanomaterial is carried out the test of scanning electron microscope among the embodiment 1, and the result is as follows figure 1 As shown, the microstructure of Co-B / NGO composite nanomaterials is a lamellar structure.
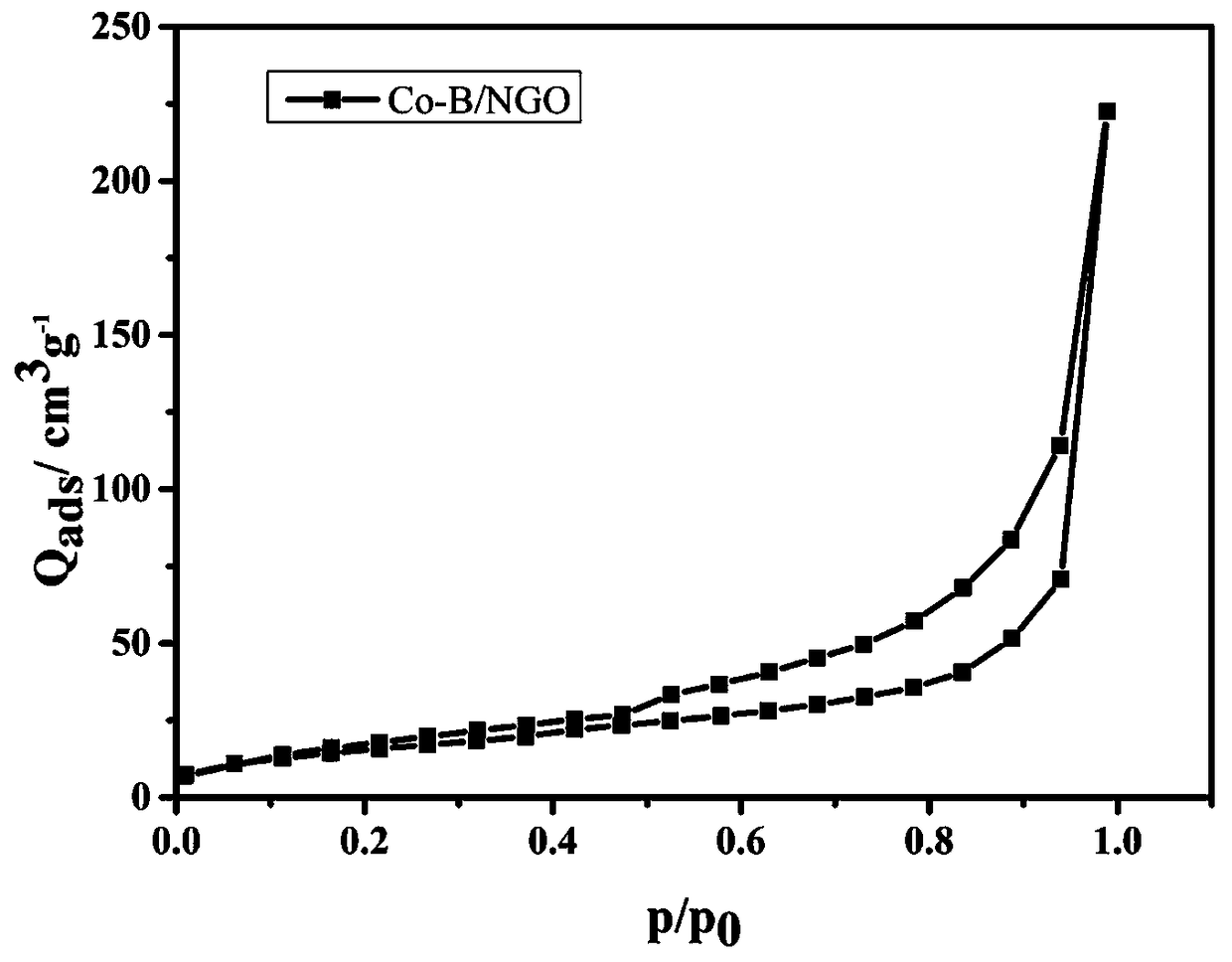
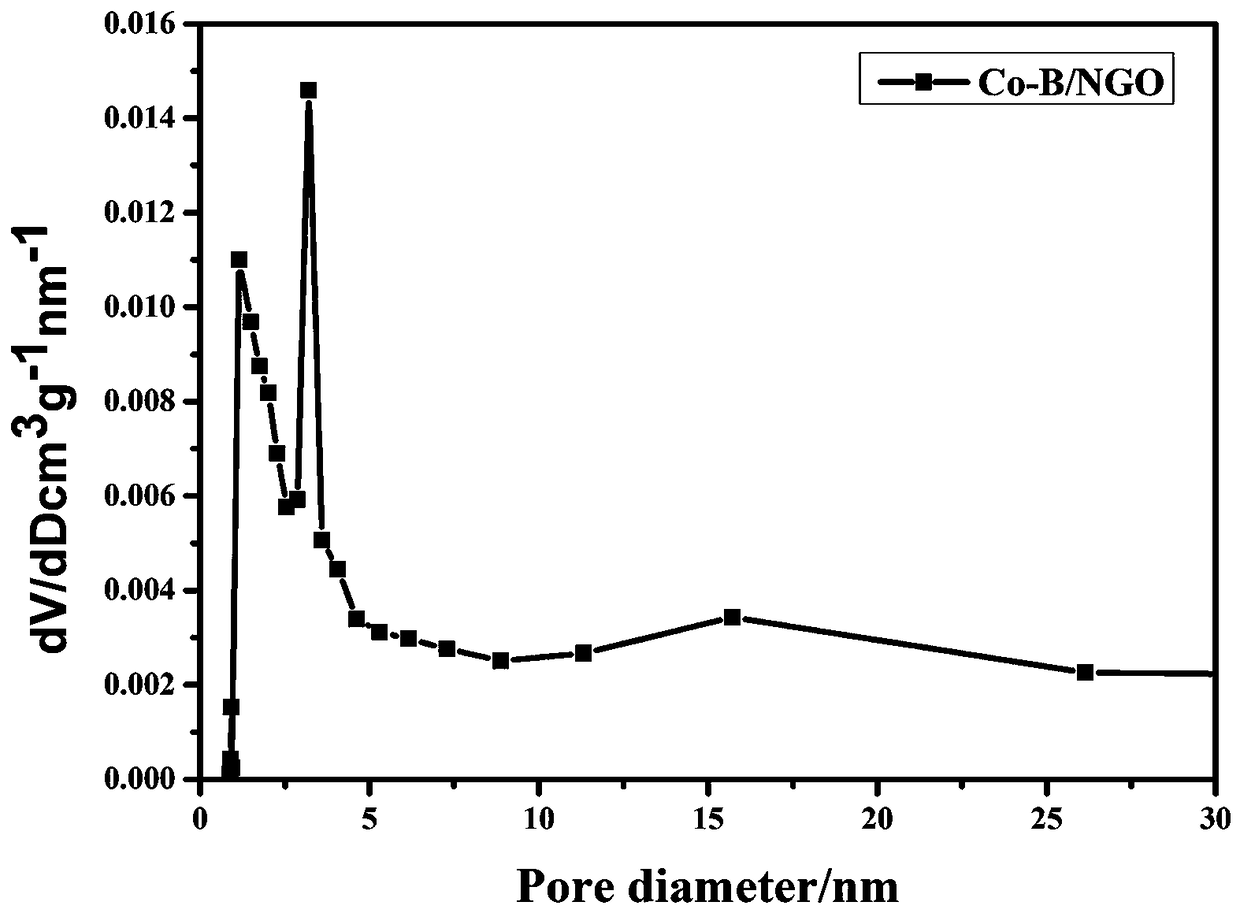
[0036] The Co-B / NGO composite nanomaterial in Example 1 was tested for isothermal adsorption performanc...
Embodiment 2
[0042] The preparation method of Co-B / NGO nano-composite material, the concrete operation steps not specified in particular are identical with above-mentioned embodiment 1, and difference is: described step 1) ratio of the amount of substance of nitrogen-doped graphene and inorganic cobalt salt For 2: (1-6).
[0043] The characterization test methods are the same as those in Example 1 above.
[0044] The Co-B / NGO composite nanomaterial in Example 2 was tested by a scanning electron microscope, and the microstructure of the Co-B / NGO composite nanomaterial is a lamellar structure.
[0045] The Co-B / NGO composite nanomaterial in Example 2 was subjected to a low-temperature nitrogen isothermal adsorption performance test, and the Co-B / NGO composite nanomaterial specific surface area was 62m 2 g -1 .
[0046] The Co-B / NGO composite nanomaterial in Example 2 was subjected to a low-temperature nitrogen isothermal adsorption performance test, and the Co-B / NGO composite nanomaterial...
Embodiment 3
[0050] The preparation method of Co-B / NGO nano-composite material, the concrete operation steps not specified in particular are identical with above-mentioned embodiment 1, and difference is: described step 1) ratio of the amount of substance of nitrogen-doped graphene and inorganic cobalt salt For 3: (1-6).
[0051] The characterization test methods are the same as those in Example 1 above.
[0052] The Co-B / NGO composite nanomaterial in Example 3 was tested by a scanning electron microscope, and the microstructure of the Co-B / NGO composite nanomaterial is a lamellar structure.
[0053] The Co-B / NGO composite nanomaterial in Example 3 was subjected to a low-temperature nitrogen isothermal adsorption performance test, and the Co-B / NGO composite nanomaterial specific surface area was 59m 2 g -1 .
[0054] The Co-B / NGO composite nanomaterial in Example 3 was subjected to a low-temperature nitrogen isothermal adsorption performance test, and the Co-B / NGO composite nanomaterial...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com