Method for producing long-chain alkanes by hydrothermal reduction of carbon dioxide with iron powder and cobalt powder
A technology for long-chain alkanes and carbon dioxide, applied in chemical instruments and methods, from carbon oxides to hydrocarbons, metal/metal oxides/metal hydroxide catalysts, etc., can solve problems such as rapid catalyst deactivation, and avoid transportation , Huge stock and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, commercial iron powder and cobalt powder reduction carbon dioxide are alkane
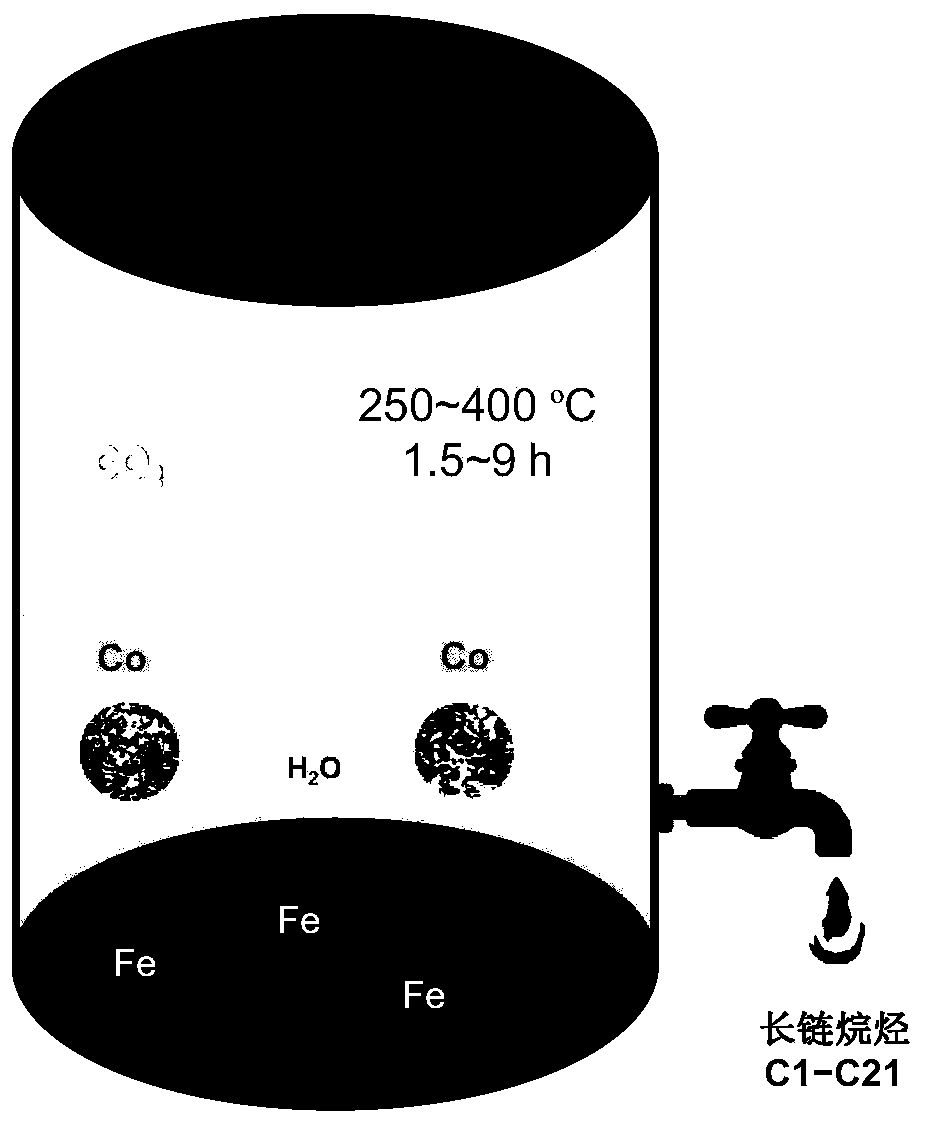
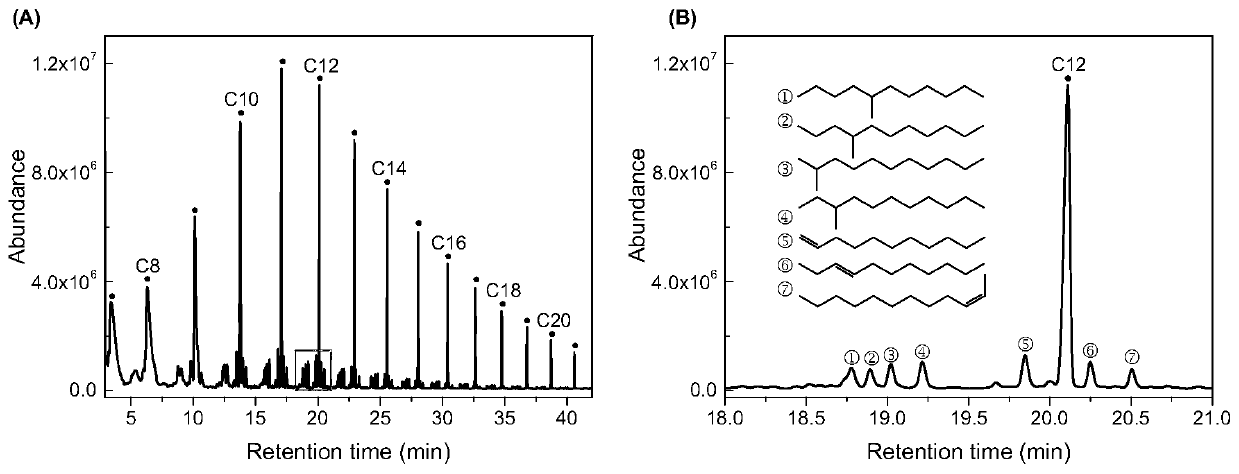
[0039] Such as figure 1 As shown, iron powder, cobalt powder and sodium bicarbonate are added to the batch reaction kettle according to the amount of substances of 100mmol, 200mmol and 400mmol, and then water is added, and the filling rate of the container is 40%. After the reaction kettle was sealed, it was placed in an induction heating furnace, and reacted at 250°C for 6 hours, and the system pressure was 20Mpa. The reacted gas mainly contains methane, ethane and propane. Dichloromethane was added to the liquid to extract long-chain alkanes. The yield of alkanes was ~1%, with a selectivity of 3-6%. The extracted liquid contains organic acids such as formic acid and acetic acid, and the corresponding chemicals can be obtained through fractional distillation.
[0040] figure 2 It is the scanning electron micrograph contrast figure of cobalt catalyst before and after rea...
Embodiment 2
[0042] Embodiment 2, commercial iron powder and cobalt powder reduction carbon dioxide are alkane
[0043] Iron powder, cobalt powder and sodium bicarbonate were added into the batch reaction kettle according to the amount of substances of 40mmol, 4mmol and 20mmol, and then water was added, and the filling rate of the container was 10%. After the reaction kettle was sealed, it was placed in an induction heating furnace, and reacted at 150° C. for 12 hours, and the system pressure was 1 Mpa. The reacted gas mainly contains methane, ethane and propane. Dichloromethane was added to the liquid to extract long-chain alkanes. The yield of alkanes was ~1%, with a selectivity of 3-6%. The extracted liquid contains organic acids such as formic acid and acetic acid, and the corresponding chemicals can be obtained through fractional distillation.
[0044] Figure 4 is the kinetic curve of the reaction, given by Figure 4 It can be seen that the reaction rate is relatively slow bef...
Embodiment 3
[0045] Embodiment 3, commercial iron powder and cobalt powder reduction carbon dioxide are alkane
[0046] Iron powder, cobalt powder and sodium bicarbonate are added into the batch reaction kettle according to the amount of substances 40mmol, 400mmol and 400mmol, and then water is added, and the filling rate of the container is 80%. After the reaction kettle was sealed, it was placed in an induction heating furnace, and reacted at 400°C for 0.5h, and the system pressure was 42Mpa. The reacted gas mainly contains methane, ethane and propane. Dichloromethane was added to the liquid to extract long-chain alkanes. The yield of alkanes was ~1%, with a selectivity of 3-6%. The extracted liquid contains organic acids such as formic acid and acetic acid, and the corresponding chemicals can be obtained through fractional distillation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com