Fluorenyl-similar windmill-shaped nano-grid as well as preparation method and application thereof
A pinwheel-like, fluorene-like technology is applied in the field of soluble cyclic non-planar fluorene-like pinwheels and their synthesis, which can solve the problems that non-fullerene small molecule materials have not been reported, and achieve the effect of good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Cyclopentadithienyl Pinwheel I
[0059]
[0060] 220ml of dried dichloromethane was added to the reaction flask, and cyclopentadithienyl tertiary alcohol-1 (0.2g, 0.42mM) was fully dissolved in a constant pressure dropping funnel filled with 200ml of dried dichloromethane, Measure boron trifluoride ether (0.1ml) with a syringe and inject it into the reaction flask, open the valve of the constant pressure dropping funnel and add it dropwise to the reaction flask, stir for 4h, until the reaction substrate is completely reacted, add water to quench and neutralize reaction. Extract with dichloromethane, combine the dichloromethane extracts of the organic phase, dry over anhydrous magnesium sulfate, filter off the desiccant, and evaporate the solvent under reduced pressure. Cyclopentadithienyl pinwheel I as a solid (0.0729 g, 38%).
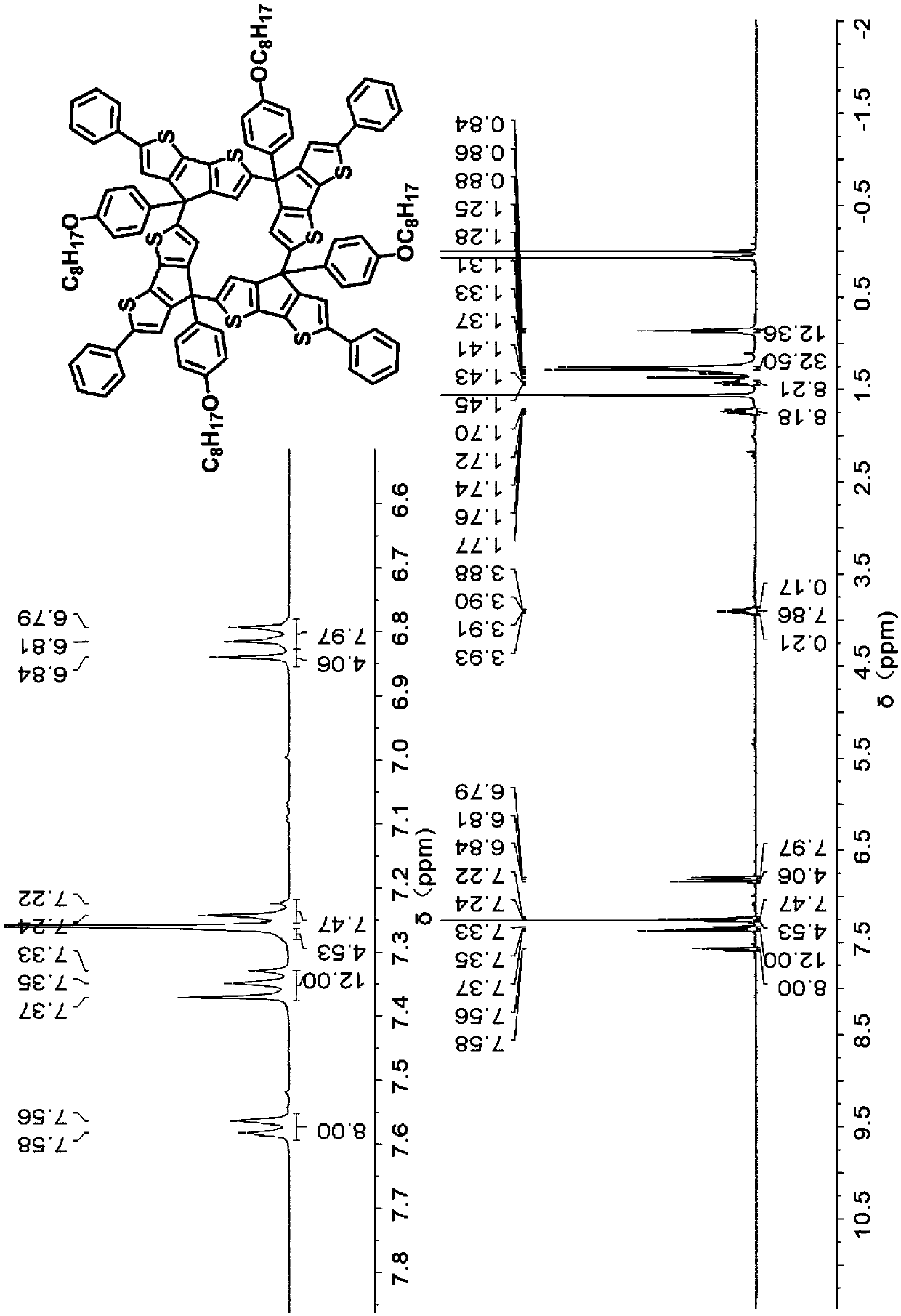
[0061] 1 H NMR (400MHz, CD 3 Cl)δ7.58-7.56(d,J=7.2Hz,8H),7.37(s,4H),7.37-7.33(t,J=8.8Hz,4H),7.28-7.22(m,6H),6.84( s, 4H), 6....
Embodiment 2
[0062] Example 2: Cyclopentadithienyl Pinwheel II
[0063]
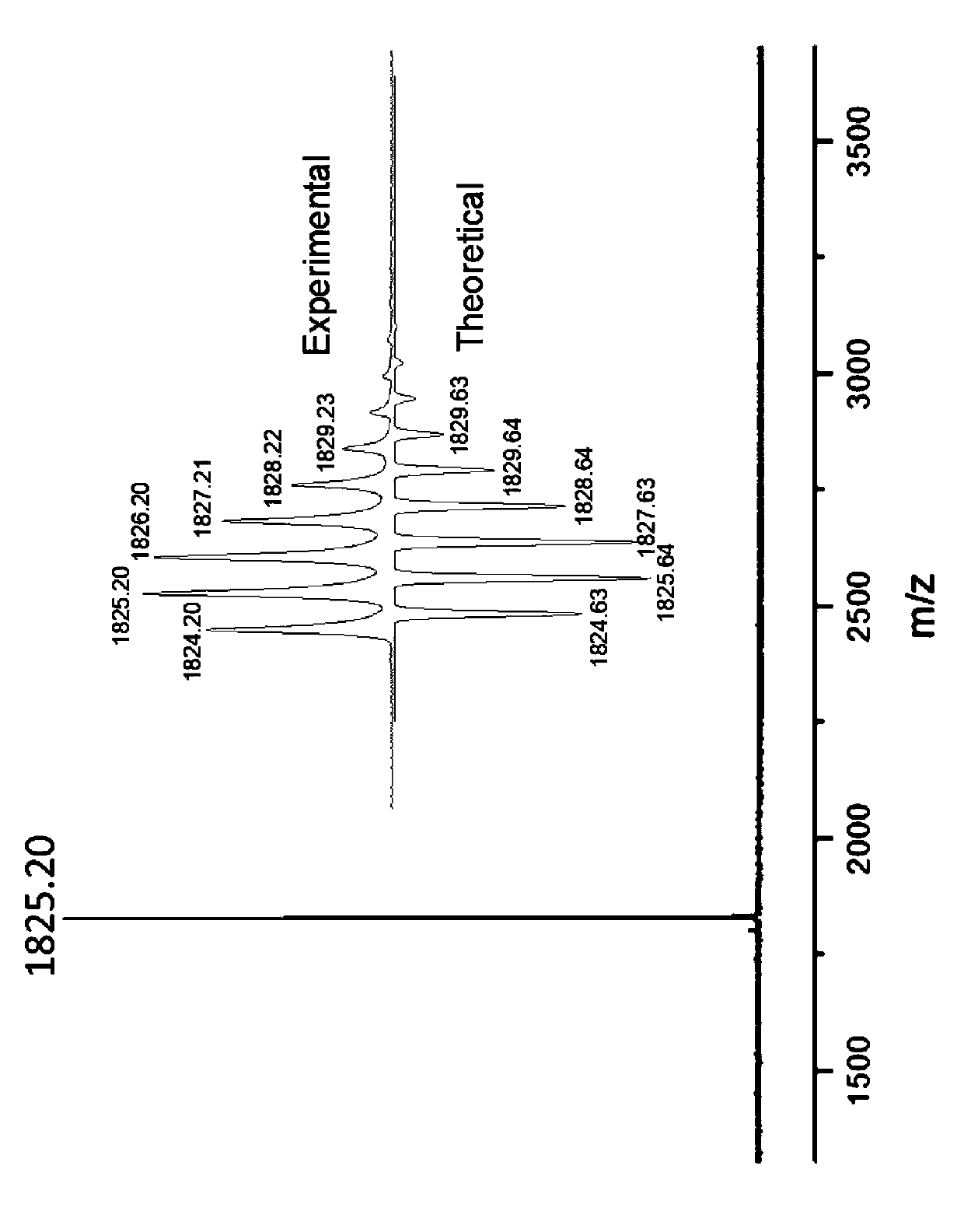
[0064] 250ml of dried dichloromethane was added to the reaction flask, and cyclopentadithienyl tertiary alcohol-2 (0.8g, 2.3mM) was fully dissolved in a constant pressure dropping funnel filled with 200ml of dried dichloromethane, Measure boron trifluoride ether (0.2ml) with a syringe and inject it into the reaction flask, open the valve of the constant pressure dropping funnel and add it dropwise into the reaction flask, stir and react for 4h, until the reaction substrate reacts completely, add water to quench and neutralize reaction. Extract with dichloromethane, combine the dichloromethane extracts of the organic phase, dry over anhydrous magnesium sulfate, filter off the desiccant, and evaporate the solvent under reduced pressure. Cyclopentadithienyl pinwheel II as a solid (0.272 g, 36%).
[0065] 1 H NMR (400MHz, CD 3 Cl)δ7.59-7.57(d,J=7.6,8H),7.40(s,4H),7.38-7.30(m,24H),7.10-7.07(dd,J=8.4,8H),6.98(s, 4H...
Embodiment 3
[0067] Electronic energy levels of cyclopentadithienyl pinwheel II were measured by electrochemical cyclic voltammetry, where Ag / AgNO 3 As reference electrode, Fc / Fc + pair as an internal reference. According to the initial oxidation potential (E ox ) and the onset reduction potential (E red ), the lowest unoccupied molecular orbital (LUMO) energy level (E LUMO ) and the highest occupied molecular orbital (HOMO) energy level (E HOMO ) can be obtained by Equation E LUMO / HOMO =-e(E red / ox +4.682) (eV) OK. (In our measurement system, the redox potential of Fc / Fc+ relative to Ag / AgNO 3 0.118V, we set the vacuum level of Fc / Fc+ to be 4.8eV. ) The LUMO and HOMO energy levels of cyclopentadithienyl pinwheel II are -3.54 and -5.85 eV, respectively.
[0068] Therefore, the fluorene-like pinwheel-like nanolattice will have a good application prospect in the field of organic solar cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com