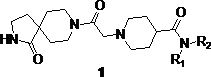
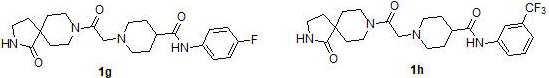
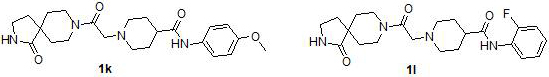Preparation and application of diazaspirodecane piperidine carboxamides
A technology of diazaspiro and decanepiperidine, which is applied in the field of medicine and can solve problems such as threats to human health, increased morbidity and mortality of fungal infections, and low immunity of the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、8
[0029] Example 1, Preparation of 8-(2-chloroacetyl)-2,8-diazaspiro[4.5]dec-1-one 4
[0030] Add compound 2 (0.62 g, 4.0 mmol), anhydrous potassium carbonate (0.66 g, 4.8 mmol), dry dichloromethane (15 mL) into a 50 mL round bottom flask, stir at room temperature for 30 min, and add chlorine dropwise in an ice bath Acetyl chloride was a solution of compound 3 (0.4 mL, 4.8 mmol) in dichloromethane (15 mL). Stir overnight at room temperature, then reflux for 30 min, cool to room temperature, add 5% sodium bicarbonate solution (20 mL), stir for 10 min, extract with dichloromethane (25 mL×2), combine organic layers, anhydrous sulfuric acid Sodium drying, suction filtration, vacuum concentration, and column chromatography yielded compound 4 (0.39 g), white powder, yield 42.5%, melting point: 149.7-151.1°C; 1 H NMR (600 MHz, CDCl 3 ) δ 6.62 (s, 1H, CONH), 4.14-4.02 (m, 2H, CH 2 ), 4.01-3.81 (m, 2H, diazaspiro-CH 2 ), 3.35-3.25 (m, 4H, diazaspiro-CH 2 ),2.05-1.96 (m, 2H, diazaspi...
Embodiment 2
[0031] Embodiment 2, the preparation of N-(2-methoxyphenyl)piperidine-4-carboxamide (7a)
[0032] Add 1-(tert-butoxycarbonyl)piperidine-4-carboxylic acid, compound 5 (2.35 g, 10.2 mmol), pyridine (2.1 mL, 26.0 mmol), dichloromethane (30 mL ), filled with nitrogen protection, added thionyl chloride (0.9 mL, 12.4 mmol) at room temperature, continued to stir for 30 min, and slowly added dropwise a solution containing o-methoxyaniline (1.42 g, 11.5 mmol), triethylamine (4.9 mL, 35.3 mmol), a catalytic amount of DMAP in dichloromethane solution (30 mL), react at room temperature for 10 h after the dropwise addition, and then add 1 mol / mL hydrochloric acid (30 mL×2), saturated sodium bicarbonate (30 mL ×2) Wash, dry with anhydrous sodium sulfate, filter with suction, concentrate in vacuo, and obtain the intermediate (2.47 g) after column chromatography, add the intermediate (2.47 g) into (50 mL) ethyl acetate, stir and dissolve , add 15% hydrochloric acid (2.5 mL), react at 50°C fo...
Embodiment 3
[0034] Example 3, N-(2-methoxyphenyl)-1-(2-oxo-2-(1-oxo-2,8-diazaspiro[4.5]decane-8-yl) Preparation of ethyl)piperidine-4-carboxamide (1a)
[0035] In a 25 mL round bottom flask, add compound 7a (0.42 g, 1.8 mmol), ground potassium carbonate (0.25 g, 1.8 mmol), catalytic amount of KI (0.07 g, 0.4 mmol), acetonitrile 10 mL, and stir at room temperature After 30 min, compound 4 (0.35 g, 1.5 mmol) was added, heated to 70°C for 8 h, filtered, concentrated in vacuo, and compound 1a (0.25 g) was obtained after column chromatography, a white powder, yield 39.6%, melting point: 136.3-137.5°C; 1 H NMR (600 MHz, CDCl 3 ) δ 8.30 (d, J =7.9 Hz, 1H, CONH), 7.78 (s, 1H, Ar-H), 6.96 (t, J =7.7 Hz, 1H, Ar-H), 6.88 (t, J =7.7 Hz, 1H, Ar-H), 6.80 (d, J =8.1 Hz, 1H, Ar-H), 6.31 (s, 1H,CONH), 3.82(s, 3H, Ar-OCH 3 ), 3.31-3.06 (m, 6H, diazaspiro-CH 2 ), 2.90 (d, J =10.8 Hz, 2H, CH 2 ), 2.20 (dd, J =16.1, 6.9 Hz, 1H, piperidine-CH), 2.12-1.97 (m, 4H, piperidine-CH 2 ), 1.95-1.74 (m, 8H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com