Electrolyte solution and secondary battery
An electrolyte, dinitrile-based technology, applied in secondary batteries, secondary battery repair/maintenance, organic electrolytes, etc., can solve problems such as insufficient film-forming capacity, reduction, and inability to more effectively reduce cell gas production, etc. To achieve the effect of improving gas production and inhibiting side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Battery production:
[0018] Positive electrode preparation: the positive electrode active material LiNi 0.8 co 0.1 mn 0.1 o 2 (Lithium nickel cobalt manganese) and conductive agent acetylene black (SuperP) are mixed evenly in a stirring tank, then N-methylpyrrolidone (NMP) and binder polyvinylidene fluoride glue (PVDF) are added to it and stirred evenly , obtain a kind of black slurry, be coated on the aluminum foil, after baking, roll pressing, obtain the positive electrode sheet after cutting into pieces, wherein the mass ratio of positive electrode active material, conductive agent, binding agent is (92:5:3 ).
[0019] Negative electrode preparation: Mix the negative electrode active material graphite and the conductive agent acetylene black (SuperP) in a stirring tank, then add the binder SBR and deionized water into it, and stir evenly to obtain a black slurry, which is coated on the copper Foil, baked, rolled, and cut into pieces to obtain the negative elec...
Embodiment 2~10
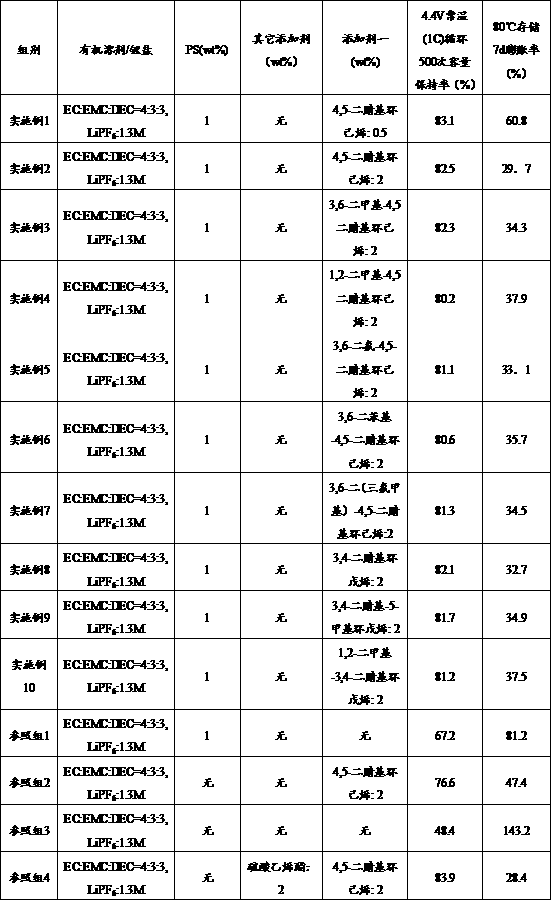
[0023] In Examples 2-10 and reference groups 1-4, except that the composition content of the electrolyte is added as shown in Table 1, the others are the same as in Example 1. Table 1 is the contents of each component of the electrolyte and battery performance test results of Examples 1-10 and reference groups 1-4:
[0024] Cycle test: The batteries obtained in Examples 1-10 and reference groups 1-4 were subjected to a charge-discharge cycle test at 25°C at a charge-discharge rate of 1C / 1C in the range of 2.8-4.4V, and the first discharge of the battery was recorded Capacity and discharge capacity after each cycle, cycle 500 cycles, capacity retention = discharge capacity per cycle / first discharge capacity of the battery*100%, the recorded data is shown in Table 1.
[0025] Volume expansion experiment: charge the battery 1C obtained in Examples 1-10 and reference groups 1-4 to 4.4V, measure the volume by the drainage method, record the initial volume and the volume after 7 day...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com