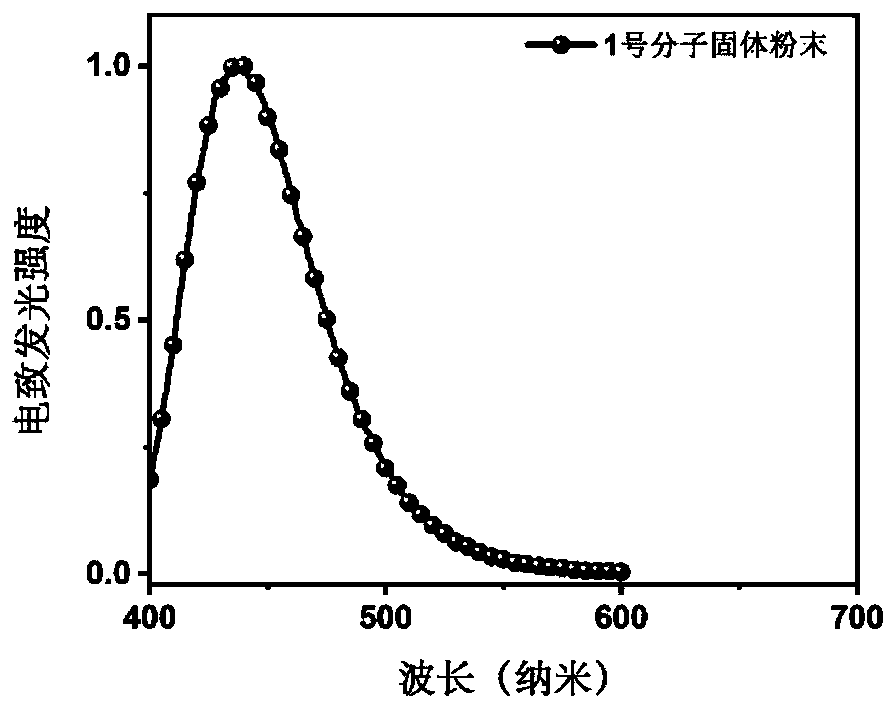
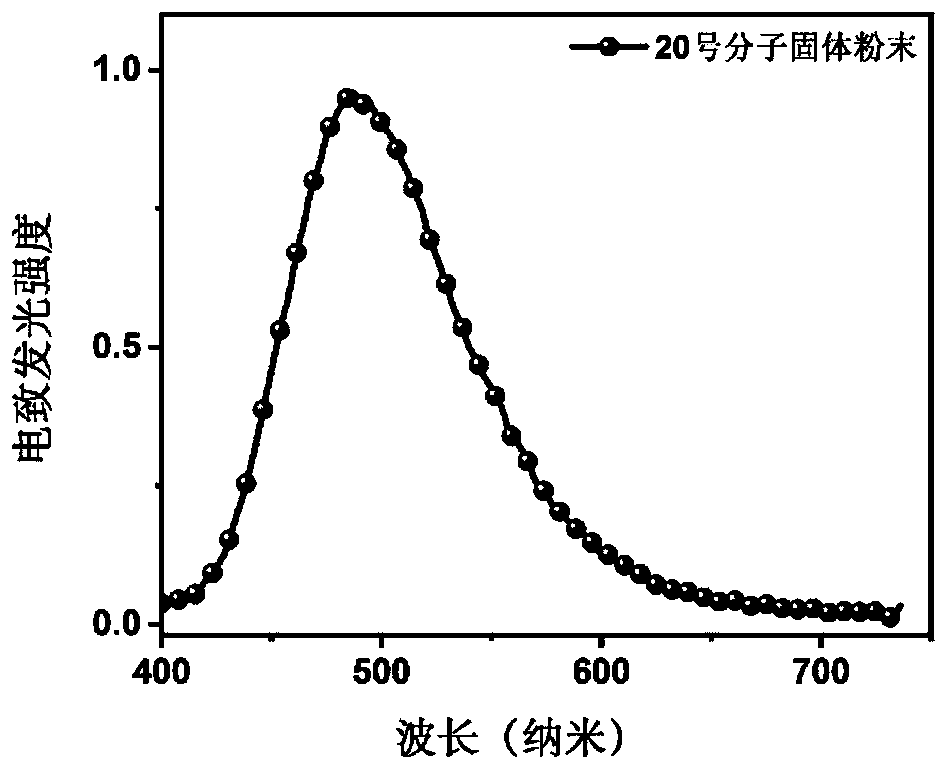
Benzonitrile derivative and application thereof to production of electroluminescent device
A derivative, benzonitrile technology, applied in the field of benzonitrile derivatives and its application in the preparation of electroluminescent devices, can solve the problem that the luminescent color cannot be adjusted, and achieve excellent electroluminescence performance and good current carrying Sub-transmission characteristics, the effect of wide adjustment range of luminous peak position
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0166] Embodiment 1: the synthesis of compound 1
[0167] Add 10 mmol of compound 1a, 15 mmol of diphenylamine, and 30 mmol of potassium tert-butoxide into a two-necked flask under nitrogen atmosphere, and add 50 mL of ultra-dry DMF under nitrogen atmosphere. Reflux reaction at about 160°C for 12h. After the reaction was completed, after the temperature of the system dropped to room temperature, it was poured into 1 L of ice water, stirred thoroughly, and filtered with suction. The filter cake was washed with deionized water and methanol three times in turn, and then dried. Using 200-300 mesh silica gel as the stationary phase, dichloromethane:petroleum ether=1:3 (volume ratio) as the eluent for column chromatography separation, the white powder that is product 1 can be obtained. The molecular ion mass determined by mass spectrometry is: 600.2 (calculated value: 600.2); theoretical element content (%) C 43 h 28 N 4 : C, 85.97; H, 4.70; N, 9.33; Measured element content (%...
Embodiment 2
[0169] Embodiment 2: the synthesis of compound 2
[0170] According to the reaction conditions and process of Example 1, using 1a and 4,4'-dimethyldiphenylamine as raw materials, white solid compound 2 was obtained. The molecular ion mass determined by mass spectrometry is: 628.2 (calculated value: 628.3); theoretical element content (%) C 45 h 32 N 4 : C, 85.96; H, 5.13; N, 8.91; Measured element content (%): C, 85.96; H, 5.13; N, 8.91.
Embodiment 3
[0171] Embodiment 3: the synthesis of compound 3
[0172] According to the reaction conditions and process of Example 1, using 1a and 4,4'-diisopropyldiphenylamine as raw materials, white solid compound 3 was obtained. The molecular ion mass determined by mass spectrometry is: 684.3 (calculated value: 684.3); theoretical element content (%) C 49 h 40 N 4 : C, 85.93; H, 5.89; N, 8.18; Measured element content (%): C, 85.93; H, 5.89; N, 8.18.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com