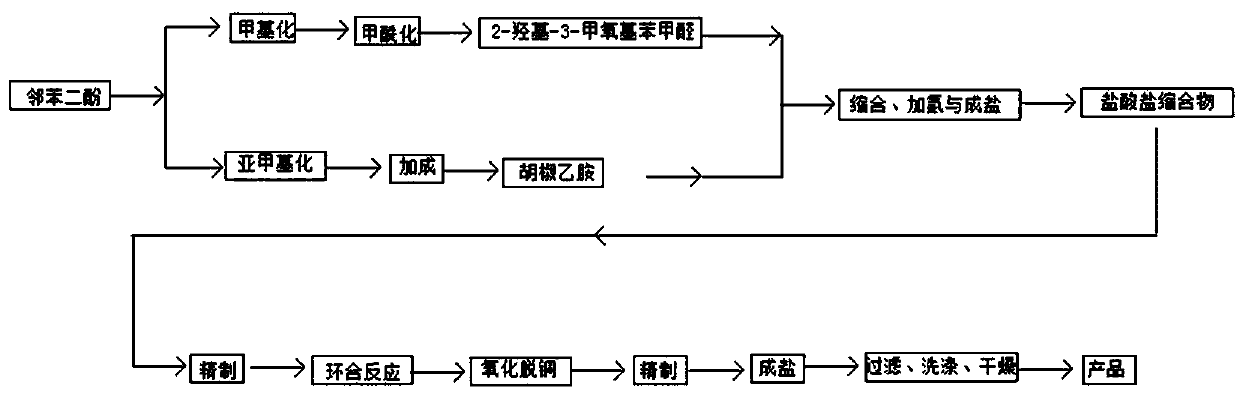
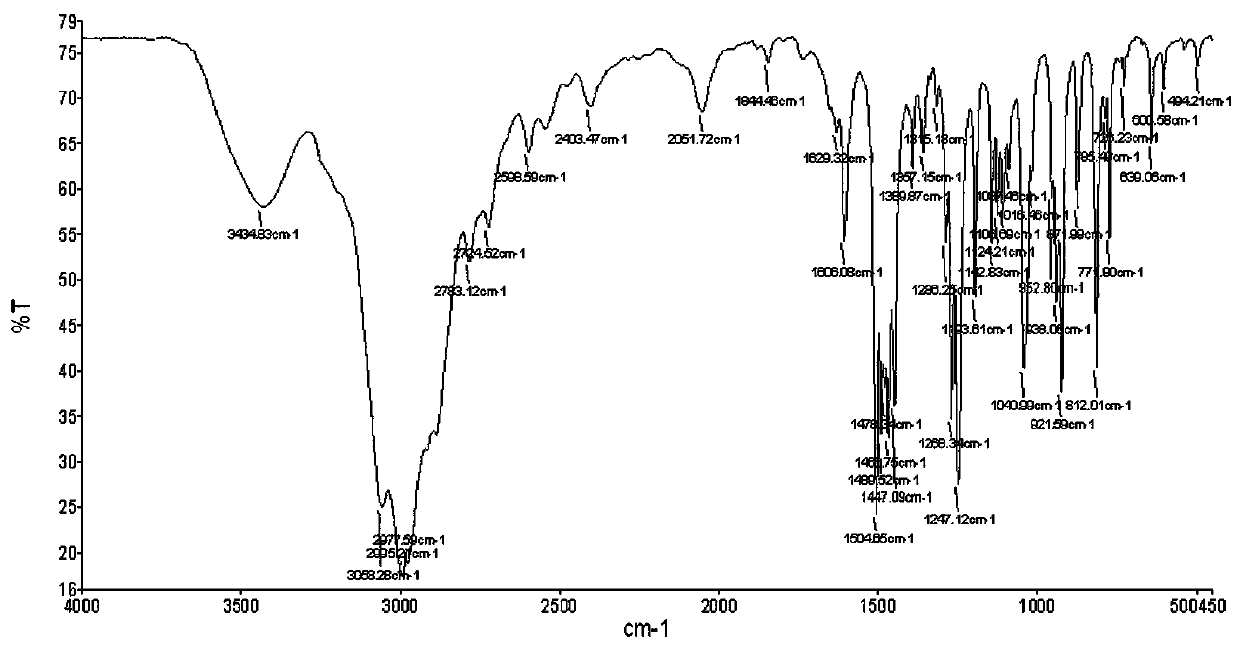
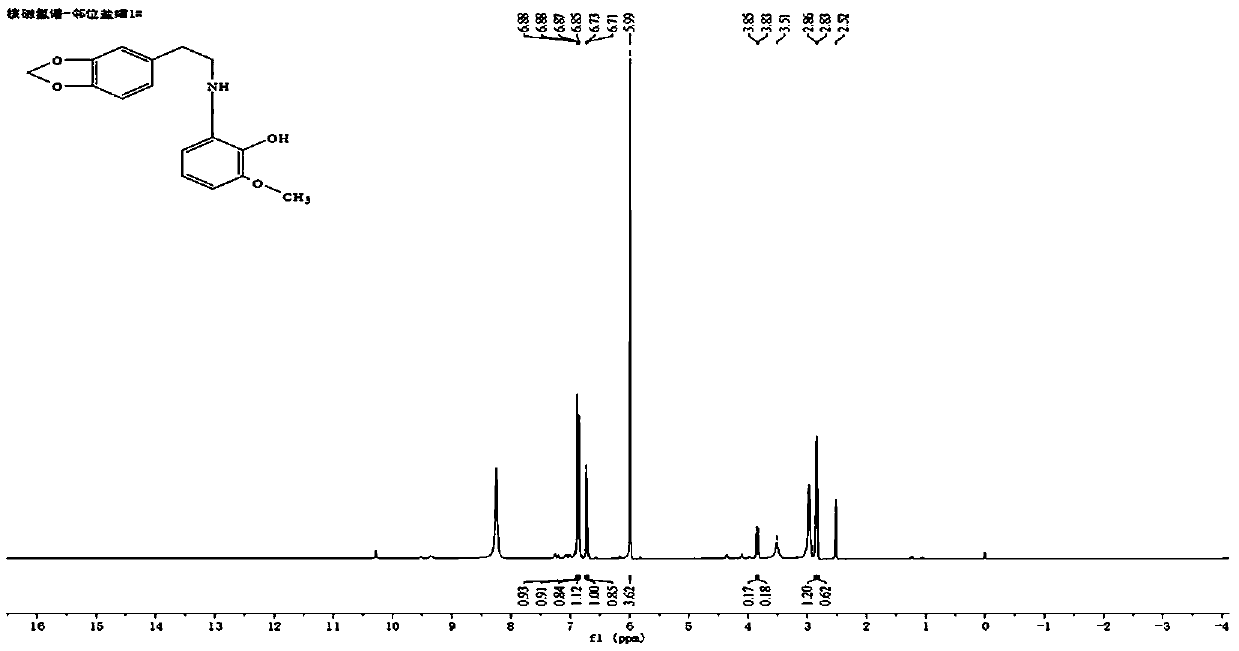
Method for totally synthesizing berberrubine from catechol
A technology of catechol and berberine, which is applied in the field of full synthesis of berberine, can solve the problems of unfavorable expansion of the use of berberine drugs, consumption of berberine production, and consumption of berberine drugs, etc. Achieve significant economic and social benefits, avoid toxic cyanide, and reduce the pain of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0047] (1) Weigh catechol and 2-chloromethane according to the molar ratio, put them into a 500ml three-necked flask with dimethyl sulfoxide as the solvent, add an acid-binding agent (alkali), react at 130°C for 6 hours, reduce Part of the 2-chloromethane was recovered by pressure distillation, and the product at 130-135°C was collected to obtain the target product piperonine.
[0048] (2) Using ethyl acetate as a solvent, weigh piperonylcycline and 2-chloroethylamine according to the molar ratio, put them into a 500ml three-neck flask, add catalyst, add 2-chloroethylamine dropwise, stir for 8 hours, and after cooling, reduce Ethyl acetate was obtained by pressing, crystallized at -10°C, vacuum filtered, and recrystallized from ethanol to obtain piperonylethylamine.
[0049] (3) According to the mass ratio of 1:4, weigh catechol and sodium hydroxide, put them into a 500ml three-necked flask, add catalysts (alcohols), use methanol as a solvent, heat up and stir until the solid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com