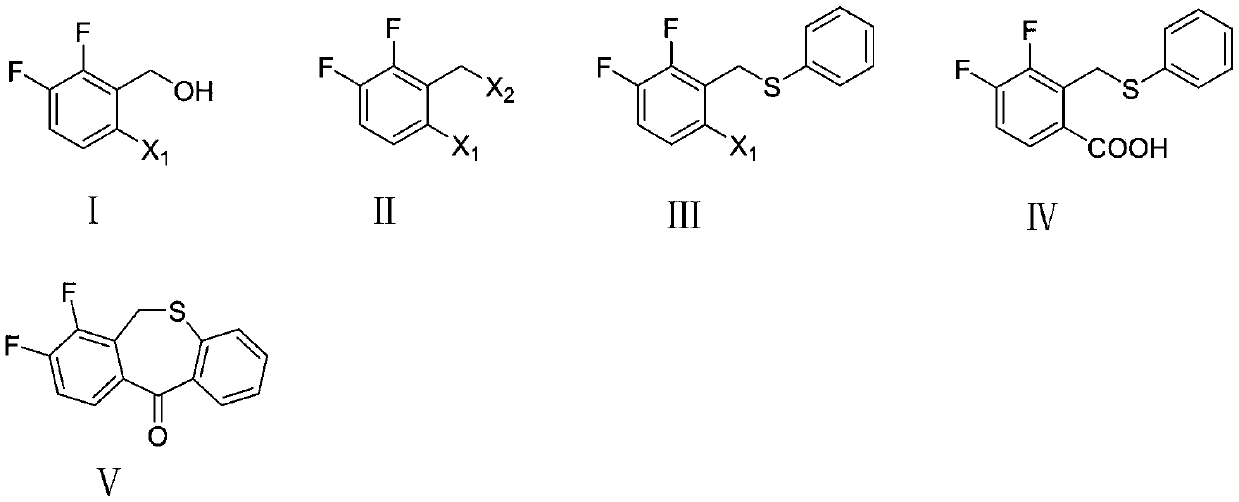
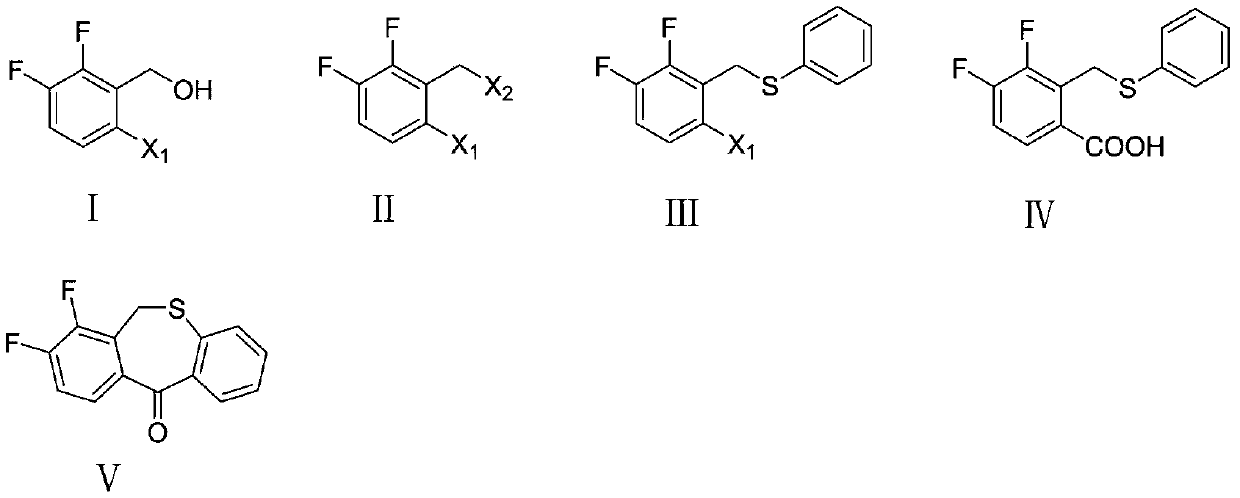
Preparation method of key intermediate of baloxavir marboxil
An intermediate and key technology are applied in the field of preparation of key intermediates of baloxavir, can solve problems such as incapable replacement of vaccination, and achieve the effects of less production risk factors, simple and convenient post-processing, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of 1-bromo-2-(bromomethyl)-3,4-difluorobenzene
[0034] Add (6-bromo-2,3-difluorophenyl)methanol (4.46g, 20mmol), 40% hydrobromic acid (8.1g, 40mmol), and 70mL of acetonitrile into a 250ml two-necked flask, and stir the reaction at room temperature 4 hour, TLC tracked to after the completion of the reaction, added water to the reaction solution (the volume of water and the reaction solution was the same), then added ethyl acetate for extraction, left to separate into organic phase and water phase, and the organic phase was washed with water and dried Afterwards, it was concentrated under reduced pressure to remove acetonitrile and ethyl acetate solvents to obtain 5.12 g of brown-yellow liquid with a yield of 89.5%.
[0035] 1 H NMR (600MHz, Chloroform-d) δ7.35 (ddd, J = 8.3, 4.1, 1.9Hz, 1H), 7.06 (q, J = 8.3Hz, 1H), 4.62 (q, J = 1.6Hz, 2H) . 13C NMR (101MHz, Chloroform-d) δ150.81(dd, J=54.0, 13.4Hz), 148.30(dd, J=59.0, 13.4Hz), 128.43(dd, J=6.1...
Embodiment 2
[0036] Example 2: Preparation of 1-bromo-2-(bromomethyl)-3,4-difluorobenzene
[0037] Add (6-bromo-2,3-difluorophenyl)methanol (4.46g, 20mmol), phosphorus tribromide (8.12g, 30mmol) and 70mL dry ether into a 250ml two-necked flask, and stir the reaction at 0°C After 4 hours, TLC tracked to the completion of the reaction, poured the reaction solution into ice water, added ethyl acetate for extraction, left to separate into an organic phase and an aqueous phase, washed the organic phase with water, dried, and concentrated under reduced pressure to remove ethyl acetate solvent to obtain 3.32 g of brown-yellow liquid with a yield of 58.1%.
[0038] 1 H NMR (600MHz, Chloroform-d) δ7.35 (ddd, J = 8.3, 4.1, 1.9Hz, 1H), 7.06 (q, J = 8.3Hz, 1H), 4.62 (q, J = 1.6Hz, 2H) . 13 C NMR (101MHz, Chloroform-d) δ150.81(dd, J=54.0, 13.4Hz), 148.30(dd, J=59.0, 13.4Hz), 128.43(dd, J=6.1, 4.5Hz), 127.74(d , J=13.2Hz), 118.98 (dd, J=3.8, 1.9Hz), 118.35 (d, J=18.1Hz), 24.64 (dd, J=4.5, 2.4Hz).
Embodiment 3
[0039] Example 3: Preparation of 1-bromo-2-(bromomethyl)-3,4-difluorobenzene
[0040] Add (6-bromo-2,3-difluorophenyl)methanol (4.46g, 20mmol), 40% hydrobromic acid (8.1g, 40mmol), and 70mL ethyl acetate into a 250ml two-necked flask, and stir at room temperature Reacted for 4 hours, after TLC tracked to the completion of the reaction, water was added to the reaction solution (the volume of water and the reaction solution was the same), then ethyl acetate was added for extraction, and the standing layering was divided into an organic phase and an aqueous phase, and the organic phase was washed with water, After drying, it was concentrated under reduced pressure to remove the ethyl acetate solvent to obtain 4.3 g of a brownish-yellow liquid with a yield of 75%.
[0041] 1 H NMR (600MHz, Chloroform-d) δ7.35 (ddd, J = 8.3, 4.1, 1.9Hz, 1H), 7.06 (q, J = 8.3Hz, 1H), 4.62 (q, J = 1.6Hz, 2H) . 13 C NMR (101MHz, Chloroform-d) δ150.81(dd, J=54.0, 13.4Hz), 148.30(dd, J=59.0, 13.4Hz),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com