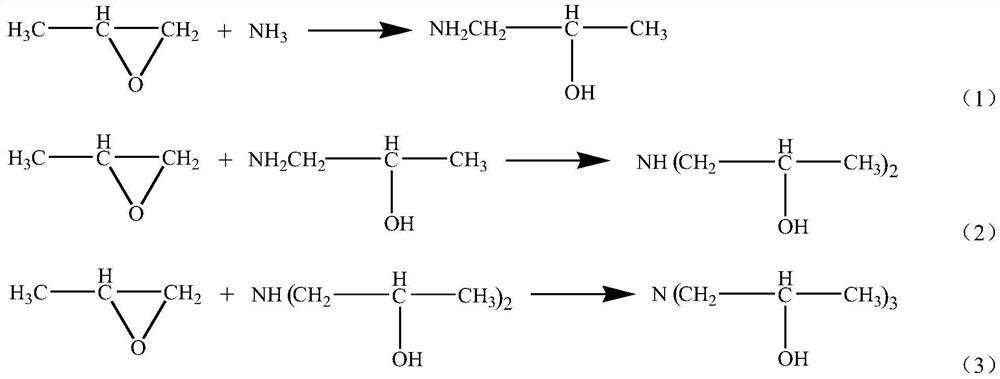
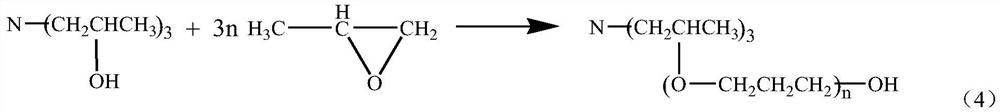
The production method of isopropanolamine
A technology of isopropanolamine and production method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as the influence of product purity, reduce the probability of polymerization, reduce energy consumption, and improve product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
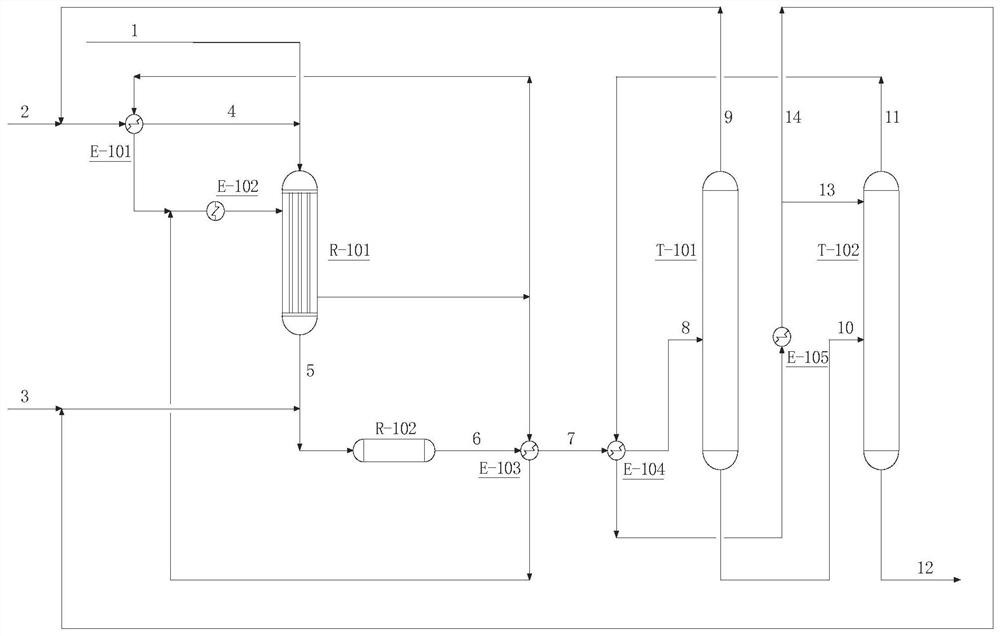
[0037] Fresh liquid ammonia feed 2 is mixed with circulating liquid ammonia 9 and sent to E-101. After being preheated by circulating hot water in reactor R-101, liquid ammonia feed 4 is mixed with propylene oxide feed 1 and sent to R-101 , to obtain the primary reaction product flow 5, after mixing with fresh water 3 and circulating ammonia water 14, send it to R-102, the outlet of R-102 is decompressed, and the depressurized reaction product 6 is first preheated in E-103 by circulating hot water , 7 is sent to E-104, after 11 preheating, 8 is sent to ammonia recovery tower T-101, the top of T-101 gets circulating liquid ammonia 9 returns to E-101, and the tower kettle gets isopropanolamine containing water and a small amount of ammonia 10 and 10 are sent to the dehydration tower T-102, and after the dehydration tower top gas 11 is condensed in E-104 and E-105, part of it returns to the top of the dehydration tower T-102 as the dehydration tower reflux 13, and part of it retur...
Embodiment 2
[0046] Fresh liquid ammonia feed 2 is mixed with circulating liquid ammonia 9 and sent to E-101. After being preheated by circulating hot water in reactor R-101, liquid ammonia feed 4 is mixed with propylene oxide feed 1 and sent to R-101 , to obtain the primary reaction product flow 5, after mixing with fresh water 3 and circulating ammonia water 14, send it to R-102, the outlet of R-102 is decompressed, and the depressurized reaction product 6 is first preheated in E-103 by circulating hot water , 7 is sent to E-104, after 11 preheating, 8 is sent to ammonia recovery tower T-101, the top of T-101 gets circulating liquid ammonia 9 returns to E-101, and the tower kettle gets isopropanolamine containing water and a small amount of ammonia 10 and 10 are sent to the dehydration tower T-102, and after the dehydration tower top gas 11 is condensed in E-104 and E-105, part of it returns to the top of the dehydration tower T-102 as the dehydration tower reflux 13, and part of it retur...
Embodiment 3
[0055] Fresh liquid ammonia feed 2 is mixed with circulating liquid ammonia 9 and sent to E-101. After being preheated by circulating hot water in reactor R-101, liquid ammonia feed 4 is mixed with propylene oxide feed 1 and sent to R-101 , to obtain the primary reaction product flow 5, after mixing with fresh water 3 and circulating ammonia water 14, send it to R-102, the outlet of R-102 is decompressed, and the depressurized reaction product 6 is first preheated in E-103 by circulating hot water , 7 is sent to E-104, after 11 preheating, 8 is sent to ammonia recovery tower T-101, the top of T-101 gets circulating liquid ammonia 9 returns to E-101, and the tower kettle gets isopropanolamine containing water and a small amount of ammonia 10 and 10 are sent to the dehydration tower T-102, and after the dehydration tower top gas 11 is condensed in E-104 and E-105, part of it returns to the top of the dehydration tower T-102 as the dehydration tower reflux 13, and part of it retur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com