Biimidazole derivatives modified modified by triphenylethylene and preparation and application of biimidazole derivatives
A technology of triphenylethylene and biimidazole, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of weak light emission and unfavorable application of fluorescent sensing materials, and achieve low preparation cost, low cost, The effect of convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
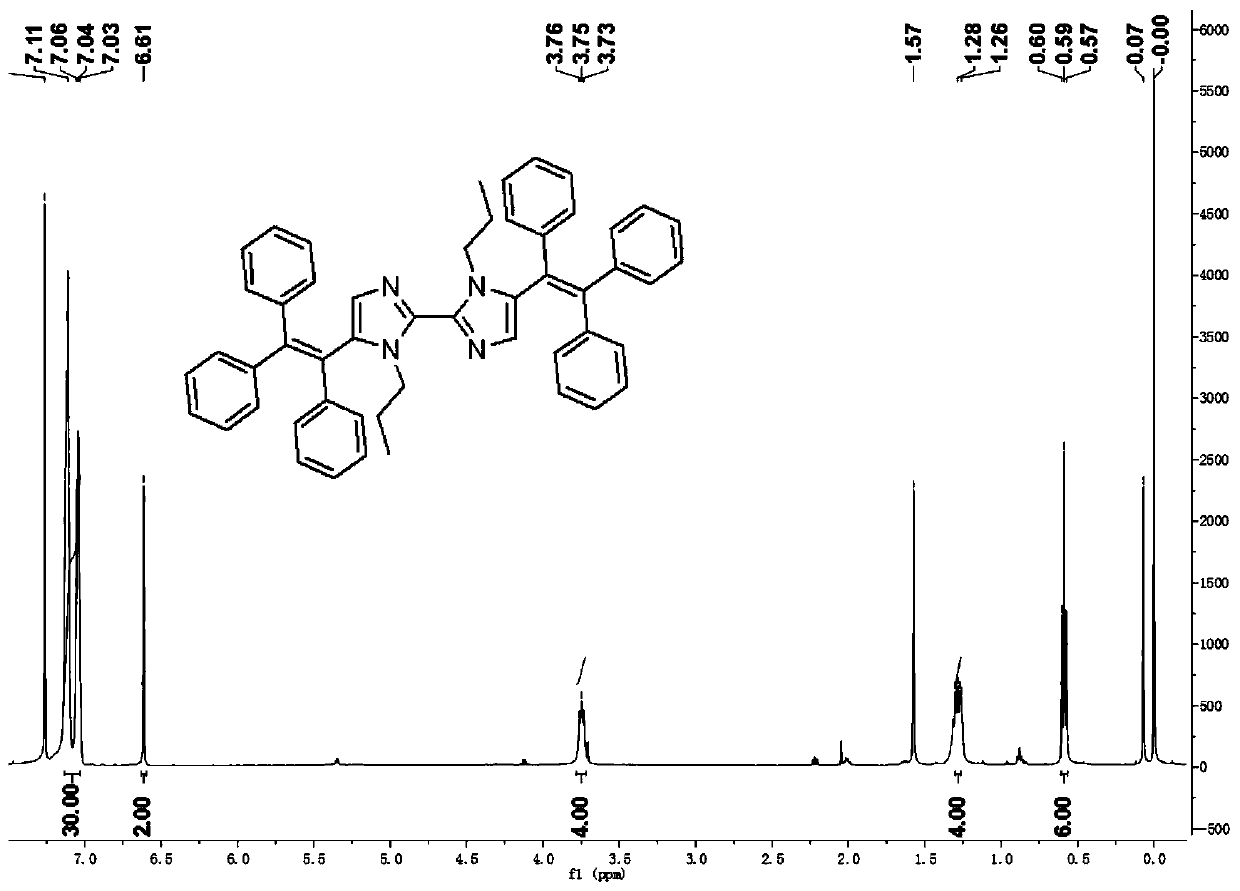
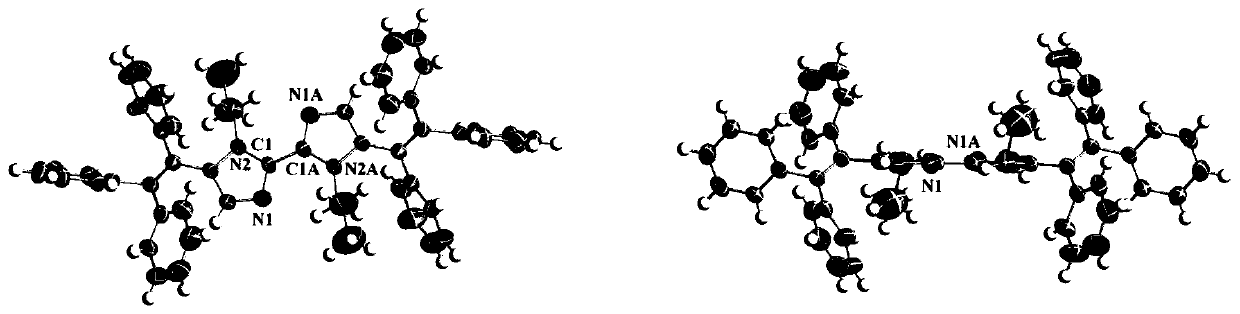
[0029] Weigh 0.10g (0.26mmol) of 5,5'-dibromo-1,1'-di-n-propyl-1H,1'H-2,2'-biimidazole, mix it with 10ml 1,4-di Put oxyhexane and 2ml of water into a 50mL flask for dissolution, then add 0.22g (0.58mmol) of 4,4,5,5-tetramethyl-2-(1,2- 2-triphenylethenyl)-1,3-dioxaborane, 0.22 g (0.66 mmol) of cesium carbonate and 30.7 mg (0.026 mmol) of catalyst tetrakis (triphenylphosphine) palladium, at 95° C. The reaction was stirred slowly under gas protection for 24 hours. 5,5'-dibromo-1,1'-di-n-propyl-1H,1'H-2,2'-biimidazole The molar ratio of 2-(1,2-2-triphenylethenyl)-1,3-dioxaborane to cesium carbonate was 10:20:25. When the reaction is over, 1 / 2 of the 1,4-dioxane in the flask is removed by a rotary evaporator, solids are precipitated, and a filter cake is obtained after filtration, using ethyl acetate / petroleum at a volume ratio of 10:1 Purified by ether column chromatography and further purified by recrystallization to obtain a yellow solid, namely 1,1'-di-n-propyl-5'5'-bis(1,2,2...
Embodiment 2
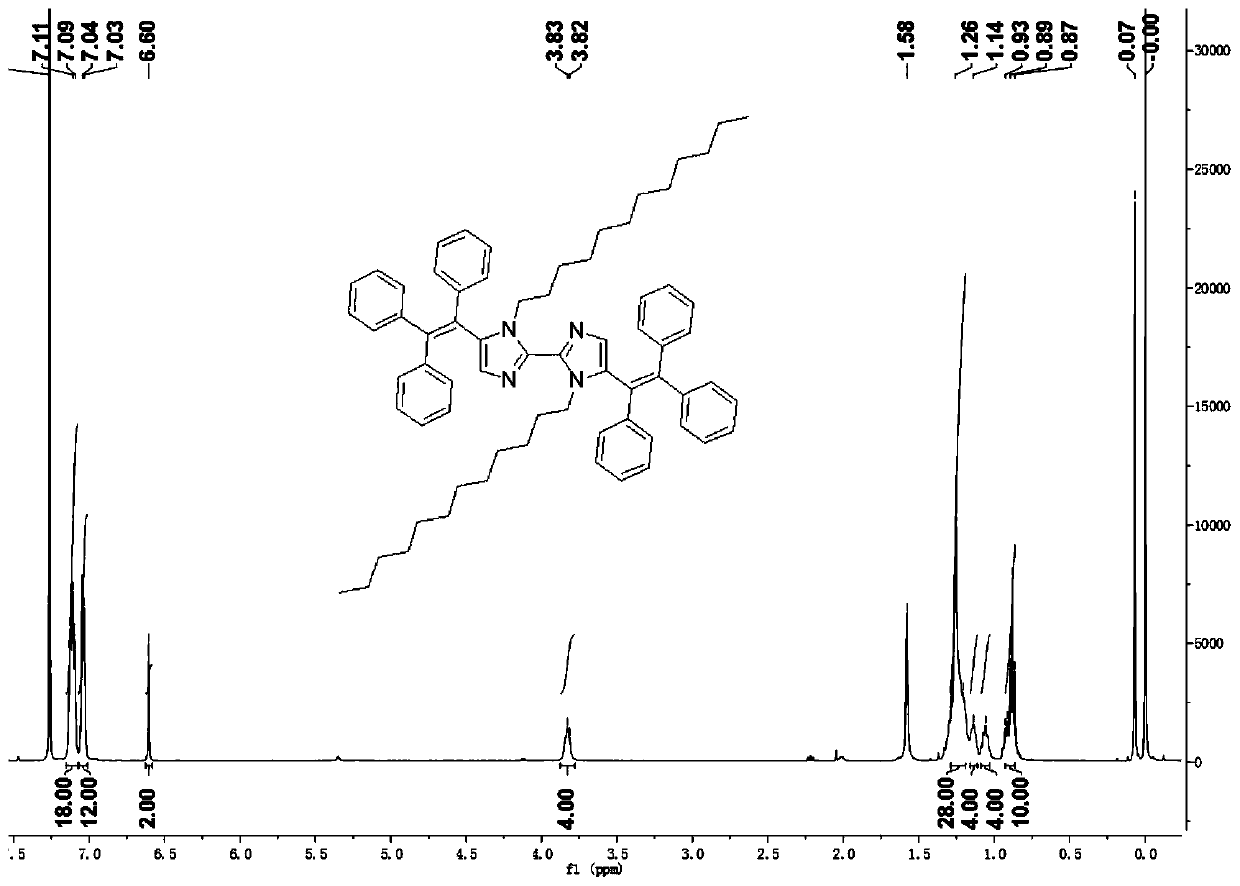
[0039] The preparation method is similar to Example 1, except that the alkyl chains are different. Weigh 0.16g (0.26mmol) of 5,5'-dibromo-1,1'-di-n-dodecyl-1H,1'H-2,2'-biimidazole, and 10ml 1,4-di Oxyhexane and 2ml of water were loaded into a 50mL flask, and 0.22g (0.58mmol) of 4,4,5,5-tetramethyl-2-(1,2-2-triphenyl Vinyl)-1,3-dioxaborane, 0.22g (0.66mmol) of cesium carbonate and 30.7mg (0.026mmol) of catalyst tetrakis (triphenylphosphine) palladium, under the protection of argon at 95°C, slowly stirred the reaction 24 hours, 5,5'-dibromo-1,1'-di-n-dodecyl-1H,1'H-2,2'-biimidazole, 4,4,5,5-tetramethyl-2- The molar ratio of (1,2-2-triphenylvinyl)-1,3-dioxaborane to cesium carbonate was 10:20:25. After the reaction is over, remove 1 / 2 of the organic solvent in the reaction system, filter, take the filter cake, use ethyl acetate / petroleum ether=10:1 column chromatography to purify and further recrystallize and purify to obtain a yellow solid, namely 1,1'-Di-n-dodecyl-5'5'-bis(1...
Embodiment 3
[0046] An application example of triphenylethylene-modified biimidazole derivatives as fluorescent probes for the detection of nitro explosives.
[0047] Step 1: Configure the density as 10 respectively -4 mol / L nitrobenzene THF solution, o-nitrotoluene THF solution, p-nitrotoluene THF solution, m-nitrotoluene THF solution, 2,6-dinitrotoluene THF solution and 2,4,6-trinitrotoluene THF solution 100 mL of phenol THF solution.
[0048] Step 2: Combine 1,1'-di-n-propyl-5'5'-bis(1,2,2-triphenylethenyl)-1H,1'H-2,2'-linked Imidazole was dissolved in anhydrous tetrahydrofuran to make 10 -4 mol / L solution (mother liquor), pipette 1.00mL mother liquor, and add 9.00mL distilled water to obtain a water content of 90% 1,1'-di-n-propyl-5'5'-bis(1,2 , 2-triphenylvinyl)-1H,1'H-2,2'-biimidazole in tetrahydrofuran. Similarly, the 1,1'-di-n-dodecyl-5'5'-bis(1,2,2-triphenylethenyl)-1H,1'H-2,2' in Example 2 - Biimidazole can also be made into 90% water content 1,1'-di-n-dodecyl-5'5'-bis(1,2,2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com